Ann Rehabil Med.
2011 Dec;35(6):747-758. 10.5535/arm.2011.35.6.747.
Facilitation of Corticospinal Excitability According to Motor Imagery and Mirror Therapy in Healthy Subjects and Stroke Patients
- Affiliations
-
- 1Department of Rehabilitation Medicine, Eulji Hospital, Eulji University School of Medicine, Seoul 139-711, Korea. md52516@hanmail.net
- 2Department of Biomedical Engineering, Keimyung University, Daegu 704-701, Korea.
- KMID: 2266799
- DOI: http://doi.org/10.5535/arm.2011.35.6.747
Abstract
OBJECTIVE
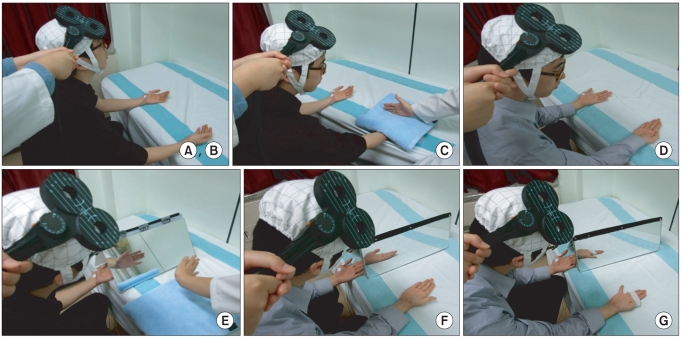
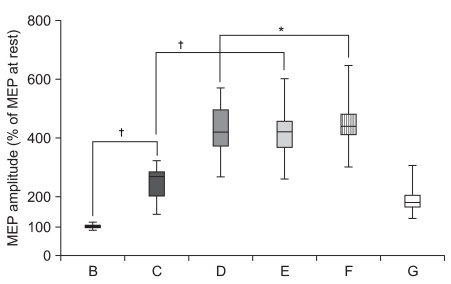
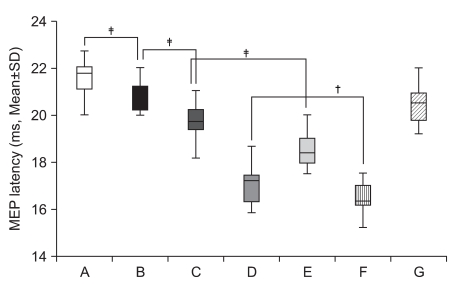
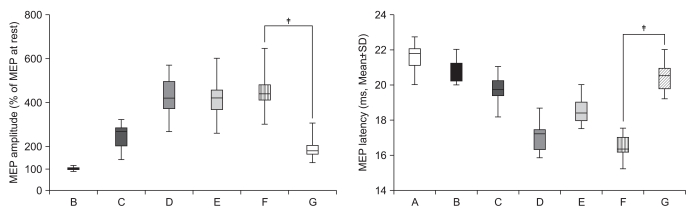
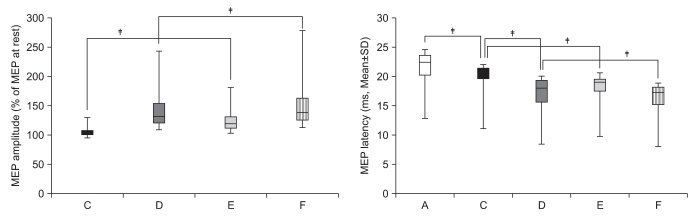
To delineate the changes in corticospinal excitability when individuals are asked to exercise their hand using observation, motor imagery, voluntary exercise, and exercise with a mirror. METHOD: The participants consisted of 30 healthy subjects and 30 stroke patients. In healthy subjects, the amplitudes and latencies of motor evoked potential (MEP) were obtained using seven conditions: (A) rest; (B) imagery; (C) observation and imagery of the hand activity of other individuals; (D) observation and imagery of own ipsilateral hand activity; (E) observation and imagery of the hand activity of another individual with a mirror; (F) observation and imagery of own symmetric ipsilateral hand activity (thumb abduction) with a mirror; and (G) observation and imagery of own asymmetric ipsilateral hand activity (little finger abduction) with a mirror. In stroke patients, MEPs were obtained in the A, C, D, E, F conditions.
RESULTS
In both groups, increment of the percentage MEP amplitude (at rest) and latency decrement of MEPs were significantly higher during the observation of the activity of the hand of another individual with a mirror and during symmetric ipsilateral hand activity on their own hand with a mirror than they were without a mirror. In healthy subjects, the increment of percentage MEP amplitude and latency decrement were significantly higher during the observation of the symmetric ipsilateral hand activity with a mirror compared to the observation of the activity of the asymmetric ipsilateral hand with a mirror of their own hand.
CONCLUSION
In both groups, corticospinal excitability was facilitated by viewing the mirror image of the activity of the ipsilateral hand. These findings provide neurophysiological evidence supporting the application of various mirror imagery programs during stroke rehabilitation.
Keyword
Figure
Cited by 1 articles
-
Virtual Reality-Guided Motor Imagery Increases Corticomotor Excitability in Healthy Volunteers and Stroke Patients
Hyungjun Im, Jeunghun Ku, Hyun Jung Kim, Youn Joo Kang
Ann Rehabil Med. 2016;40(3):420-431. doi: 10.5535/arm.2016.40.3.420.
Reference
-
1. Han CE, Arbib MA, Schweighofer N. Stroke rehabilitation reaches a threshold. PLoS Comput Biol. 2008; 4:e1000133. PMID: 18769588.
Article2. Page SJ, Sisto SA, Levine P. Modified constraint-induced therapy in chronic stroke. Am J Phys Med Rehabil. 2002; 81:870–875. PMID: 12394999.
Article3. Nudo RJ. Adaptive plasticity in motor cortex: implications for rehabilitation after brain injury. J Rehabil Med. 2003; 41(Suppl):7–10. PMID: 12817650.
Article4. Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006; 19:84–90. PMID: 16415682.
Article5. Oujamaa L, Relave I, Froger J, Mottet D, Pelissier JY. Rehabilitation of arm function after stroke. Literature review. Ann Phys Rehabil Med. 2009; 52:269–229. PMID: 19398398.
Article6. Mulder T. Motor imagery and action observation: cognitive tools for rehabilitation. J Neural Transm. 2007; 114:1265–1278. PMID: 17579805.
Article7. Sharma N, Baron JC, Rowe JB. Motor imagery after stroke: relating outcome to motor network connectivity. Ann Neurol. 2009; 66:604–616. PMID: 19938103.
Article8. Kuhtz-Buschbeck JP, Mahnkopf C, Holzknecht C, Siebner H, Ulmer S, Jansen O. Effector-independent representations of simple and complex imagined finger movements: a combined fMRI and TMS study. Eur J Neurosci. 2003; 18:3375–3387. PMID: 14686911.
Article9. Ruby P, Decety J. What you believe versus what you think they believe: a neuroimaging study of conceptual perspective-taking. Eur J Neurosci. 2003; 17:2475–2480. PMID: 12814380.
Article10. Stinear CM, Byblow WD, Steyvers M, Levin O, Swinnen SP. Kinesthetic, but not visual, motor imagery modulates corticomotor excitability. Exp Brain Res. 2006; 168:157–164. PMID: 16078024.
Article11. Page SJ, Levine P, Leonard A. Mental practice in chronic stroke: results of a randomized, placebo-controlled trial. Stroke. 2007; 38:1293–1297. PMID: 17332444.12. Verbunt JA, Seelen HA, Ramos FP, Michielsen BH, Wetzelaer WL, Moennekens M. Mental practice-based rehabilitation training to improve arm function and daily activity performance in stroke patients: a randomized clinical trial. BMC Neurol. 2008; 8:7. PMID: 18405377.
Article13. Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996; 263:377–386. PMID: 8637922.14. Dohle C, Pullen J, Nakaten A, Kust J, Rietz C, Karbe H. Mirror therapy promotes recovery from severe hemiparesis: a randomized controlled trial. Neurorehabil Neural Repair. 2009; 23:209–217. PMID: 19074686.
Article15. Yavuzer G, Selles R, Sezer N, Sütbeyaz S, Bussmann JB, Köseoğlu F, Atay MB, Stam HJ. Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2008; 89:393–398. PMID: 18295613.
Article16. Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve. 2000; 23:1761–1763. PMID: 11054757.
Article17. Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002; 125:1896–1907. PMID: 12135979.
Article18. Calautti C, Naccarato M, Jones PS, Sharma N, Day DD, Carpenter AT, Bullmore ET, Warburton EA, Baron JC. The relationship between motor deficit and hemisphere activation balance after stroke: a 3T fMRI study. Neuroimage. 2007; 34:322–331. PMID: 17045490.
Article19. Stinear CM, Barber PA, Coxon JP, Fleming MK, Byblow WD. Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain. 2008; 131:1381–1390. PMID: 18356189.
Article20. Garry MI, Loftus A, Summers JJ. Mirror, mirror on the wall: viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Exp Brain Res. 2005; 163:118–122. PMID: 15754176.
Article21. Funase K, Tabira T, Higashi T, Liang N, Kasai T. Increased corticospinal excitability during direct observation of self movement and indirect observation with a mirror box. Neurosci Lett. 2007; 419:108–112. PMID: 17481817.22. Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000; 406:147–150. PMID: 10910346.
Article23. Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994; 91:79–92. PMID: 7519144.
Article24. Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol. 2006; 117:1641–1659. PMID: 16595189.
Article25. Wassermann EM. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol. 2002; 113:1165–1171. PMID: 12088713.
Article26. Leonard G, Tremblay F. Corticomotor facilitation associated with observation, imagery and imitation of hand actions: a comparative study in young and old adults. Exp Brain Res. 2007; 177:167–175. PMID: 16947064.
Article27. Clark S, Tremblay F, Ste-Marie D. Differential modulation of corticospinal excitability during observation, mental imagery and imitation of hand actions. Neuropsychologia. 2004; 42:105–112. PMID: 14615080.
Article28. Buccino G, Solodkin A, Small SL. Functions of the mirror neuron system: implications for neurorehabilitation. Cogn Behav Neurol. 2006; 19:55–63. PMID: 16633020.
Article29. Merians AS, Tunik E, Fluet GG, Qiu Q, Adamovich SV. Innovative approaches to the rehabilitation of upper extremity hemiparesis using virtual environments. Eur J Phys Rehabil Med. 2009; 45:123–133. PMID: 19158659.30. Shinoura N, Suzuki Y, Watanabe Y, Yamada R, Tabei Y, Saito K, Yagi K. Mirror therapy activates outside of cerebellum and ipsilateral M1. NeuroRehabilitation. 2008; 23:245–252. PMID: 18560141.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Virtual Reality-Guided Motor Imagery Increases Corticomotor Excitability in Healthy Volunteers and Stroke Patients
- Effect of Motor Imagery on the F-Wave Parameters in Hemiparetic Stroke Survivors
- Motor Imagery and Action Observation
- The After-effect of Sub-threshold 10 Hz Repetitive Transcranial Magnetic Stimulation on Motor Cortical Excitability
- Effect of Mirror Therapy on the Balance, Gait and Motor Function in Patients with Subacute Stroke