Ann Rehabil Med.
2014 Feb;38(1):19-28. 10.5535/arm.2014.38.1.19.
Factors Affecting the Motor Evoked Potential Responsiveness and Parameters in Patients With Supratentorial Stroke
- Affiliations
-
- 1Department of Physical Medicine and Rehabilitation, Korea University Hospital, Korea University College of Medicine, Seoul, Korea. rmpyun@korea.ac.kr
- KMID: 2266536
- DOI: http://doi.org/10.5535/arm.2014.38.1.19
Abstract
OBJECTIVE
To investigate the factors which affect the motor evoked potential (MEP) responsiveness and parameters and to find the correlation between the function of the upper extremities and the combined study of MEP with a diffusion tensor tractography (DTT) in patients with stroke.
METHODS
A retrospective study design was used by analyzing medical records and neuroimaging data of 70 stroke patients who underwent a MEP test between June 2011 and March 2013. MEP parameters which were recorded from the abductor pollicis brevis muscle were the resting motor threshold, latency, amplitude, and their ratios. Functional variables, Brunnstrom stage of hand, upper extremity subscore of Fugl-Meyer assessment, Manual Function Test, and the Korean version of Modified Barthel Index (K-MBI) were collected together with the biographical and neurological data. The DTT parameters were fiber number, fractional anisotropy value and their ratios of affected corticospinal tract. The data were compared between two groups, built up according to the presence (MEP-P) or absence (MEP-N) of MEP on the affected hand.
RESULTS
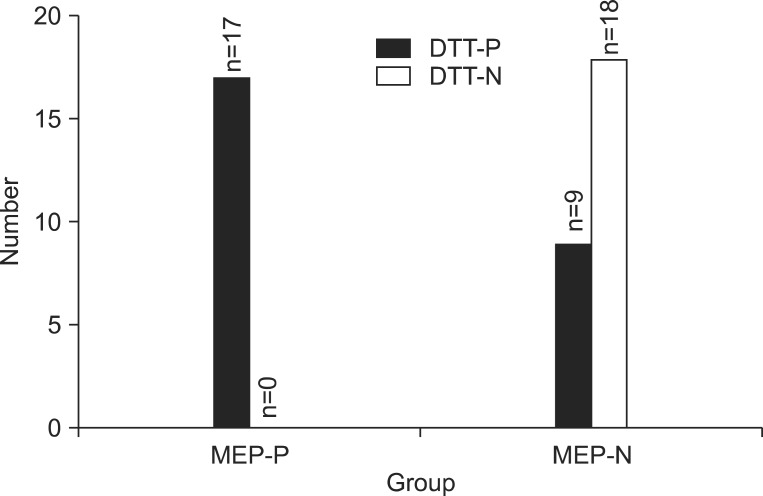
Functional and DTT variables were significantly different between MEP-P and MEP-N groups (p<0.001). Among the MEP-P group, the amplitude ratio (unaffected/affected) was significantly correlated with the Brunnstrom stage of hand (r=-0.427, p=0.013), K-MBI (r=-0.380, p=0.029) and the time post-onset (r=-0.401, p=0.021). The functional scores were significantly better when both MEP response and DTT were present and decreased if one or both of the two studies were absent.
CONCLUSION
This study indicates MEP responsiveness and amplitude ratio are significantly associated with the upper extremity function and the activities of daily living performance, and the combined study of MEP and DTT provides useful information.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Comparison of Diffusion Tensor Tractography and Motor Evoked Potentials for the Estimation of Clinical Status in Subacute Stroke
Kwang-Soo Chun, Yong-Taek Lee, Jong-Wan Park, Joon-Youn Lee, Chul-Hyun Park, Kyung Jae Yoon
Ann Rehabil Med. 2016;40(1):126-134. doi: 10.5535/arm.2016.40.1.126.Prediction of Motor Recovery Using Quantitative Parameters of Motor Evoked Potential in Patients With Stroke
Jae Yong Jo, Ahee Lee, Min Su Kim, Eunhee Park, Won Hyuk Chang, Yong-Il Shin, Yun-Hee Kim
Ann Rehabil Med. 2016;40(5):806-815. doi: 10.5535/arm.2016.40.5.806.
Reference
-
1. Young J, Forster A. Review of stroke rehabilitation. BMJ. 2007; 334:86–90. PMID: 17218714.
Article2. Shelton FN, Reding MJ. Effect of lesion location on upper limb motor recovery after stroke. Stroke. 2001; 32:107–112. PMID: 11136923.
Article3. Hendricks HT, Hageman G, van Limbeek J. Prediction of recovery from upper extremity paralysis after stroke by measuring evoked potentials. Scand J Rehabil Med. 1997; 29:155–159. PMID: 9271149.4. Escudero JV, Sancho J, Bautista D, Escudero M, Lopez-Trigo J. Prognostic value of motor evoked potential obtained by transcranial magnetic brain stimulation in motor function recovery in patients with acute ischemic stroke. Stroke. 1998; 29:1854–1859. PMID: 9731608.
Article5. Jang SH, Kim YH, Chang Y, Han BS, Byun WM, Chang CH. The predictive value of cortical activation by passive movement for motor recovery in stroke patients. Restor Neurol Neurosci. 2004; 22:59–63. PMID: 15272140.6. Nelles G, Spiekramann G, Jueptner M, Leonhardt G, Muller S, Gerhard H, et al. Evolution of functional reorganization in hemiplegic stroke: a serial positron emission tomographic activation study. Ann Neurol. 1999; 46:901–909. PMID: 10589543.
Article7. Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007; 180:583–593. PMID: 17562028.
Article8. Pennisi G, Rapisarda G, Bella R, Calabrese V, Maertens De Noordhout A, Delwaide PJ. Absence of response to early transcranial magnetic stimulation in ischemic stroke patients: prognostic value for hand motor recovery. Stroke. 1999; 30:2666–2670. PMID: 10582994.9. Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2002; 83:1629–1637. PMID: 12422337.
Article10. Han TR, Bang MS, Lee KW. Motor evoked potentials of upper and lower extremities by magnetic stimulation in hemiparesis. J Korean Acad Rehabil Med. 1998; 22:386–391.11. Kamen G. Reliability of motor-evoked potentials during resting and active contraction conditions. Med Sci Sports Exerc. 2004; 36:1574–1579. PMID: 15354040.
Article12. Christie A, Fling B, Crews RT, Mulwitz LA, Kamen G. Reliability of motor-evoked potentials in the ADM muscle of older adults. J Neurosci Methods. 2007; 164:320–324. PMID: 17588673.
Article13. Thickbroom GW, Byrnes ML, Mastaglia FL. A model of the effect of MEP amplitude variation on the accuracy of TMS mapping. Clin Neurophysiol. 1999; 110:941–943. PMID: 10400209.
Article14. Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000; 133:425–430. PMID: 10985677.
Article15. Son SY, Park SH, Seo JH, Ko MH. Correlation of the motor evoked potentials amplitude and hand function of the affected side in stroke. J Korean Acad Rehabil Med. 2011; 35:34–41.16. Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996; 111:209–219. PMID: 8661285.
Article17. Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999; 45:265–269. PMID: 9989633.
Article18. Puig J, Pedraza S, Blasco G, Daunis-I-Estadella J, Prats A, Prados F, et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol. 2010; 31:1324–1330. PMID: 20299434.
Article19. Cho SH, Kim SH, Choi BY, Cho SH, Kang JH, Lee CH, et al. Motor outcome according to diffusion tensor tractography findings in the early stage of intracerebral hemorrhage. Neurosci Lett. 2007; 421:142–146. PMID: 17566651.
Article20. Yoshioka H, Horikoshi T, Aoki S, Hori M, Ishigame K, Uchida M, et al. Diffusion tensor tractography predicts motor functional outcome in patients with spontaneous intracerebral hemorrhage. Neurosurgery. 2008; 62:97–103. PMID: 18300896.
Article21. Jang SH. Prediction of motor outcome for hemiparetic stroke patients using diffusion tensor imaging: a review. NeuroRehabilitation. 2010; 27:367–372. PMID: 21160127.
Article22. Cho SH, Kim DG, Kim DS, Kim YH, Lee CH, Jang SH. Motor outcome according to the integrity of the corticospinal tract determined by diffusion tensor tractography in the early stage of corona radiata infarct. Neurosci Lett. 2007; 426:123–127. PMID: 17897782.
Article23. Kwon YH, Son SM, Lee J, Bai DS, Jang SH. Combined study of transcranial magnetic stimulation and diffusion tensor tractography for prediction of motor outcome in patients with corona radiata infarct. J Rehabil Med. 2011; 43:430–434. PMID: 21403983.
Article24. Jang SH, Ahn SH, Sakong J, Byun WM, Choi BY, Chang CH, et al. Comparison of TMS and DTT for predicting motor outcome in intracerebral hemorrhage. J Neurol Sci. 2010; 290:107–111. PMID: 19914639.
Article25. Kunimatsu A, Aoki S, Masutani Y, Abe O, Hayashi N, Mori H, et al. The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn Reson Med Sci. 2004; 3:11–17. PMID: 16093615.
Article26. Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002; 16:232–240. PMID: 12234086.
Article27. Nakamura R. Manual function test (MFT) and functional occupational therapy for stroke patients. Tokorozawa, Japan: National Rehabilitation Center for the Disabled;2000.28. Timmerhuis TP, Hageman G, Oosterloo SJ, Rozeboom AR. The prognostic value of cortical magnetic stimulation in acute middle cerebral artery infarction compared to other parameters. Clin Neurol Neurosurg. 1996; 98:231–236. PMID: 8884095.
Article29. Miyamoto S, Kondo T, Suzukamo Y, Michimata A, Izumi S. Reliability and validity of the manual function test in patients with stroke. Am J Phys Med Rehabil. 2009; 88:247–255. PMID: 19106794.
Article30. Lim KB, Kim JA. Activity of daily living and motor evoked potentials in the subacute stroke patients. Ann Rehabil Med. 2013; 37:82–87. PMID: 23525518.
Article31. Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994; 91:79–92. PMID: 7519144.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Motor Evoked Potentials of Upper and Lower Extremities by Magnetic Stimulation in Hemiparesis
- Motor Evoked Potentials and Somatosensory Evoked Potentials of Upper and Lower Extremities for Prediction of Functional Recovery in Stroke
- The Changes of Motor Evoked Potential and Silent Period after Electrical Stimulation in Stroke Patients
- Motor evoked potential in stroke
- Motor Evoked Potential Study with Magnetic Stimulation In Ischemic Stroke Patients