Ann Dermatol.
2013 Feb;25(1):61-66. 10.5021/ad.2013.25.1.61.
The Clinical Significance of Tumor-Infiltrating Lymphocytes and Microscopic Satellites in Acral Melanoma in a Korean Population
- Affiliations
-
- 1Department of Dermatology, Kyungpook National University School of Medicine, Daegu, Korea. seokjong@knu.ac.kr
- 2Department of Pathology, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2265972
- DOI: http://doi.org/10.5021/ad.2013.25.1.61
Abstract
- BACKGROUND
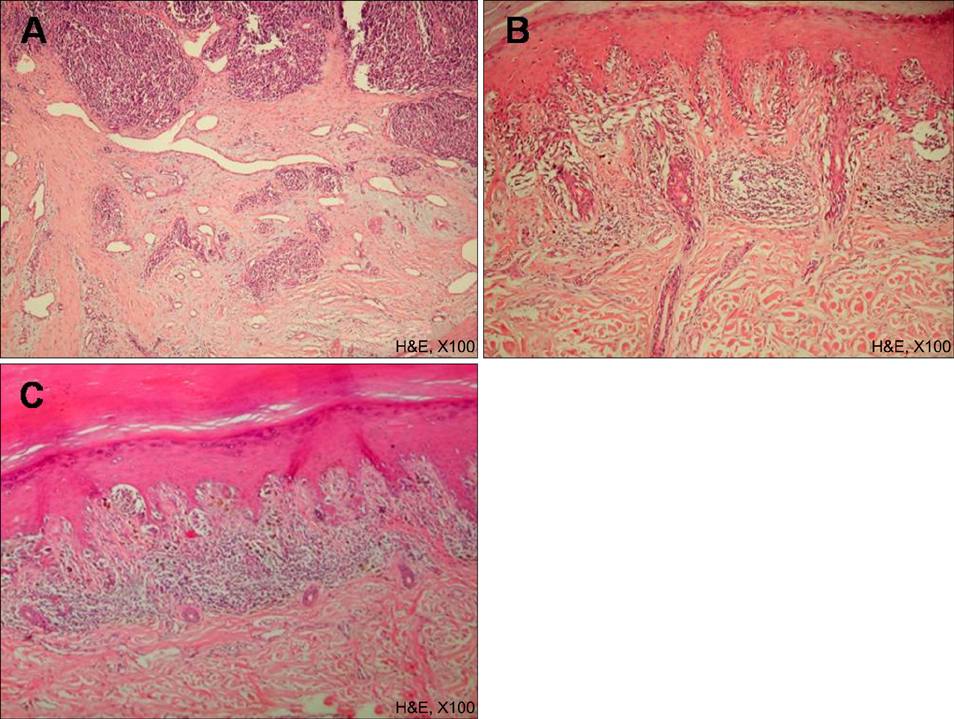
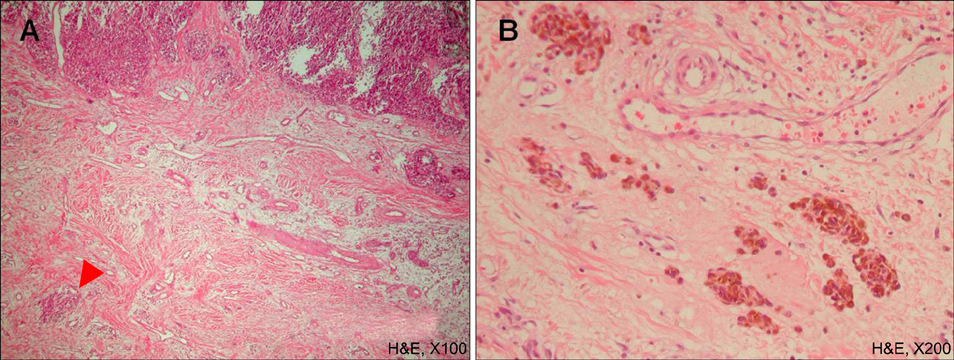
There are various histological prognostic parameters of cutaneous malignant melanoma, including tumor thickness and ulceration. Tumor-infiltrating lymphocytes (TIL) are among these parameters and can be further classified into three categories: 'absent', 'non-brisk' and 'brisk'. Brisk TIL usually indicates better clinical prognosis. Microscopic satellite (Ms) is defined as a nest of tumor cells that is greater than 0.05 mm in diameter and definitely separated from the main tumor. Even though the incidence of Ms varies according to Breslow thickness, the presence of Ms generally indicates poor prognosis.
OBJECTIVE
Clinical significance of both TIL and Ms has been extensively studied in western populations but much less so in Asian countries, including Korea, where acral melanoma is the most common subtype.
METHODS
We reviewed 90 patients with acral melanoma diagnosed at Kyungpook National University Hospital in Korea. Tissue specimens were examined using hematoxylin-eosin and HMB45 immunohistochemical staining. They were also evaluated by the presence and categorization of TIL (absent, non-brisk and brisk) and the presence of Ms. We further evaluated their impact on survival events (recurrence, distant metastasis and death).
RESULTS
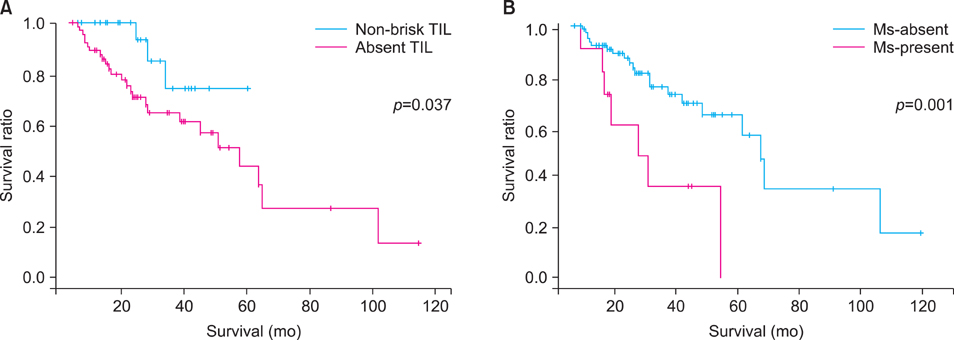
The number of survival events by TIL type was 22 in the absent category (22/64, 34.4%), 3 in the non-brisk category (3/25, 12.0%) and 0 in the brisk category. For Ms, survival events were present in 7 patients in Ms-present group (7/11, 63.6%) and 21 patients in Ms-absent group (21/79, 26.6%).
CONCLUSION
We suggest the possibility of TIL and Ms as prognostic indicators for acral melanoma in Korean population.
MeSH Terms
Figure
Reference
-
1. Nagore E, Oliver V, Botella-Estrada R, Moreno-Picot S, Insa A, Fortea JM. Prognostic factors in localized invasive cutaneous melanoma: high value of mitotic rate, vascular invasion and microscopic satellitosis. Melanoma Res. 2005. 15:169–177.
Article2. Homsi J, Kashani-Sabet M, Messina JL, Daud A. Cutaneous melanoma: prognostic factors. Cancer Control. 2005. 12:223–229.
Article3. Mihm MC Jr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996. 74:43–47.4. Busam KJ, Antonescu CR, Marghoob AA, Nehal KS, Sachs DL, Shia J, et al. Histologic classification of tumor-infiltrating lymphocytes in primary cutaneous malignant melanoma. A study of interobserver agreement. Am J Clin Pathol. 2001. 115:856–860.
Article5. Harrist TJ, Rigel DS, Day CL Jr, Sober AJ, Lew RA, Rhodes AR, et al. "Microscopic satellites" are more highly associated with regional lymph node metastases than is primary melanoma thickness. Cancer. 1984. 53:2183–2187.
Article6. León P, Daly JM, Synnestvedt M, Schultz DJ, Elder DE, Clark WH Jr. The prognostic implications of microscopic satellites in patients with clinical stage I melanoma. Arch Surg. 1991. 126:1461–1468.
Article7. Shaikh L, Sagebiel RW, Ferreira CM, Nosrati M, Miller JR 3rd, Kashani-Sabet M. The role of microsatellites as a prognostic factor in primary malignant melanoma. Arch Dermatol. 2005. 141:739–742.
Article8. Cunnon B, May JW Jr. Flynn JE, editor. Skin contractures of the hand. Hand surgery. 1982. 2nd ed. Baltimore: Williams and Wilkins;776–777.9. McGovern VJ. The nature of melanoma. A critical review. J Cutan Pathol. 1982. 9:61–81.
Article10. Clark WH Jr, Elder DE, Guerry D 4th, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989. 81:1893–1904.
Article11. Elder DE, Guerry D 4th, VanHorn M, Hurwitz S, Zehngebot L, Goldman LI, et al. The role of lymph node dissection for clinical stage I malignant melanoma of intermediate thickness (1.51-3.99 mm). Cancer. 1985. 56:413–418.
Article12. Lugassy C, Barnhill RL. Angiotropic melanoma and extravascular migratory metastasis: a review. Adv Anat Pathol. 2007. 14:195–201.13. Van Es SL, Colman M, Thompson JF, McCarthy SW, Scolyer RA. Angiotropism is an independent predictor of local recurrence and in-transit metastasis in primary cutaneous melanoma. Am J Surg Pathol. 2008. 32:1396–1403.
Article14. Sober AJ, Chuang TY, Duvic M, Farmer ER, Grichnik JM, Halpern AC, et al. Guidelines of care for primary cutaneous melanoma. J Am Acad Dermatol. 2001. 45:579–586.
Article15. Barnhill RL. Barnhill RL, editor. Prognostic factors in cutaneous melanoma. Pathology of melanocytic nevi and malignant melanoma. 1995. Boston, MA: Butterworth-Heinemann;269–284.16. Lipponen PK, Eskelinen MJ, Jauhiainen K, Harju E, Terho R. Tumour infiltrating lymphocytes as an independent prognostic factor in transitional cell bladder cancer. Eur J Cancer. 1992. 29A:69–75.
Article17. Balch CM, Riley LB, Bae YJ, Salmeron MA, Platsoucas CD, von Eschenbach A, et al. Patterns of human tumor-infiltrating lymphocytes in 120 human cancers. Arch Surg. 1990. 125:200–205.
Article18. Rilke F, Colnaghi MI, Cascinelli N, Andreola S, Baldini MT, Bufalino R, et al. Prognostic significance of HER-2/neu expression in breast cancer and its relationship to other prognostic factors. Int J Cancer. 1991. 49:44–49.19. Day CL Jr, Harrist TJ, Gorstein F, Sober AJ, Lew RA, Friedman RJ, et al. Malignant melanoma. Prognostic significance of "microscopic satellites" in the reticular dermis and subcutaneous fat. Ann Surg. 1981. 194:108–112.20. Urist MM, Balch CM, Soong S, Shaw HM, Milton GW, Maddox WA. The influence of surgical margins and prognostic factors predicting the risk of local recurrence in 3445 patients with primary cutaneous melanoma. Cancer. 1985. 55:1398–1402.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acral Lentiginous Melanoma Developing during Long-standing Atypical Melanosis: Usefulness of Dermoscopy for Detection of Early Acral Melanoma
- Treatment and Outcomes of Melanoma in Acral Location in Korean Patients
- Immunohistochemical Analysis for Basal Activation of NF-κB in Acral Lentiginous Melanoma
- Transepidermal Elimination of Nevus Cells in Acral Lentiginous Nevus
- Acral Pigmented Spitz Nevus That Clinically Mimicked Acral Lentiginous Malignant Melanoma