Ann Dermatol.
2013 Feb;25(1):5-11. 10.5021/ad.2013.25.1.5.
Preventive Effects of Multi-Lamellar Emulsion on Low Potency Topical Steroid Induced Local Adverse Effect
- Affiliations
-
- 1Department of Biology Education, Korea National University of Education, Cheongwon, Korea.
- 2Research Division, NeoPharm Co., Ltd., Daejeon, Korea.
- 3Department of Dermatology, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Dermatology, Yonsei University, Wonju College of Medicine, Wonju, Korea.
- 5Department of Dermatology, Atopy and Asthma Center, Seoul Medical Center, Seoul, Korea. caspase@hanmail.net
- KMID: 2265964
- DOI: http://doi.org/10.5021/ad.2013.25.1.5
Abstract
- BACKGROUND
Topical steroid treatment induces diverse local Wand systemic adverse effects. Several approaches have been tried to reduce the steroid-induced adverse effects. Simultaneous application of physiological lipid mixture is also suggested.
OBJECTIVE
Novel vehicles for topical glucocorticoids formulation were evaluated for the efficacy of reducing side-effects and the drug delivery properties of desonide, a low potency topical steroid.
METHODS
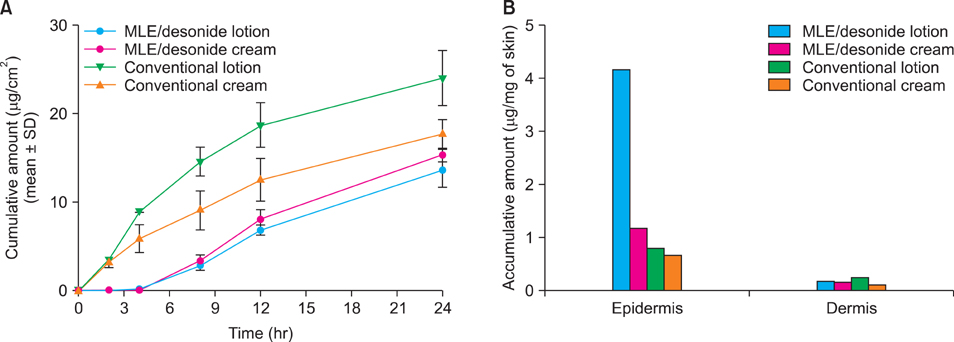
Transcutaneous permeation and skin residual amount of desonide were measured using Franz diffusion cells. The in vivo anti-inflammatory activity was evaluated using murine model.
RESULTS
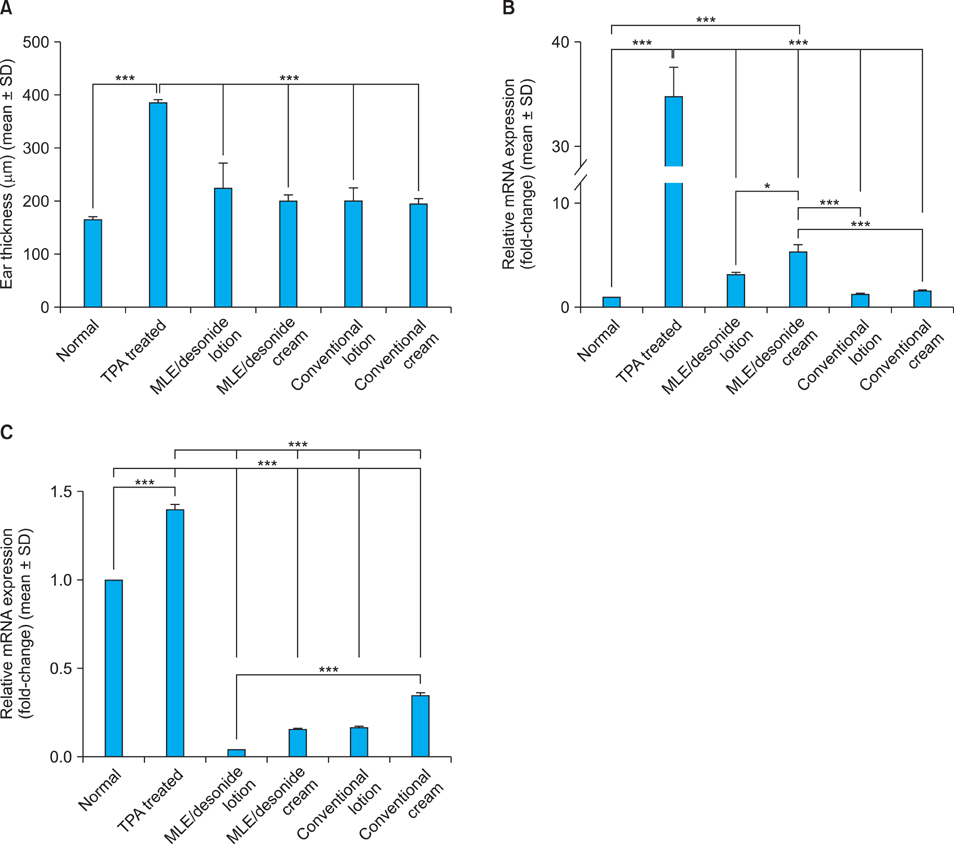
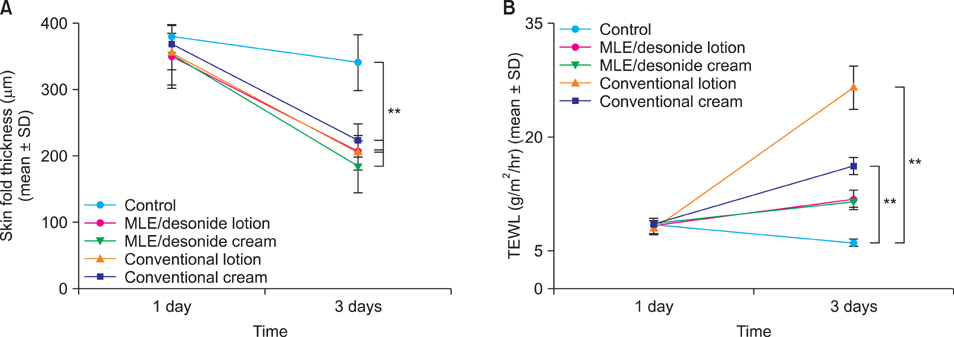
Topical steroids formulation containing desonide, in either cream or lotion form, were prepared using multi-lamellar emulsion (MLE), and conventional desonide formulations were employed for comparison. MLE formulations did not affect the anti-inflammatory activity of the desonide in phobol ester-induced skin inflammation model, compared with conventional formulations. While the penetrated amounts of desonide were similar for all the tested formulations at 24 hours after application, the increased lag time was observed for the MLE formulations. Interestingly, residual amount of desonide in epidermis was significantly higher in lotion type MLE formulation. Steroid-induced adverse effects, including permeability barrier function impairment, were partially prevented by MLE formulation.
CONCLUSION
Topical desonide formulation using MLE as a vehicle showed a better drug delivery with increased epidermal retention. MLE also partially prevented the steroid-induced side effects, such as skin barrier impairment.
Keyword
MeSH Terms
Figure
Reference
-
1. Sheu HM, Lee JY, Chai CY, Kuo KW. Depletion of stratum corneum intercellular lipid lamellae and barrier function abnormalities after long-term topical corticosteroids. Br J Dermatol. 1997. 136:884–890.
Article2. Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003. 120:456–464.
Article3. Lubach D, Rath J, Kietzmann M. Skin atrophy induced by initial continuous topical application of clobetasol followed by intermittent application. Dermatology. 1995. 190:51–55.
Article4. Lubach D, Bensmann A, Bornemann U. Steroid-induced dermal atrophy. Investigations on discontinuous application. Dermatologica. 1989. 179:67–72.5. Lubach D, Rath J, Kietzmann M. Steroid-induced dermal thinning: discontinuous application of clobetasol-17-propionate ointment. Dermatology. 1992. 185:44–48.
Article6. Korting HC, Unholzer A, Schäfer-Korting M, Tausch I, Gassmueller J, Nietsch KH. Different skin thinning potential of equipotent medium-strength glucocorticoids. Skin Pharmacol Appl Skin Physiol. 2002. 15:85–91.
Article7. Kaidbey K, Kopper SC, Sefton J, Gibson JR. A pilot study to determine the effect of tazarotene gel 0.1% on steroid-induced epidermal atrophy. Int J Dermatol. 2001. 40:468–471.
Article8. Hebert AA, Cook-Bolden FE, Basu S, Calvarese B, Trancik RJ. Desonide Hydrogel Study Group. Safety and efficacy of desonide hydrogel 0.05% in pediatric subjects with atopic dermatitis. J Drugs Dermatol. 2007. 6:175–181.9. Kircik L, Del Rosso J. A novel hydrogel vehicle formulated for the treatment of atopic dermatitis. J Drugs Dermatol. 2007. 6:718–722.10. Ahn SK, Bak HN, Park BD, Kim YH, Youm JK, Choi EH, et al. Effects of a multilamellar emulsion on glucocorticoid-induced epidermal atrophy and barrier impairment. J Dermatol. 2006. 33:80–90.
Article11. Park BD, Youm JK, Jeong SK, Choi EH, Ahn SK, Lee SH. The characterization of molecular organization of multilamellar emulsions containing pseudoceramide and type III synthetic ceramide. J Invest Dermatol. 2003. 121:794–801.
Article12. Park BD, Kim Y, Lee M, Youm JK, Jeong S, Choi EH, et al. Properties of a pseudoceramide multi-lamellar emulsion in vitro and in vivo. Cosmet Toilet. 2001. 116:65–76.13. Park BD, Lee M, Kim Y, Youm JK. Synthesis of N-ethanol-2-(myristyl/palmityl)-3-oxo(stearamide/arachidamide) and its physicla properties for a cosmetic raw material. J Cosmet Sci. 2000. 51:253–262.14. Lee EJ, Shur KB, Lee JH, Park JK, Jin CY, Youm JK, et al. The clinical efficacy of a multi-lamellar emulsion containing pseudoceramide in childhood atopic dermatitis: an open crossover study. Ann Dermatol. 2003. 15:133–138.
Article15. Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005. 125:183–200.
Article16. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002. 347:1151–1160.
Article17. Jang BC, Lim KJ, Suh MH, Park JG, Suh SI. Dexamethasone suppresses interleukin-1beta-induced human beta-defensin 2 mRNA expression: involvement of p38 MAPK, JNK, MKP-1, and NF-kappaB transcriptional factor in A549 cells. FEMS Immunol Med Microbiol. 2007. 51:171–184.
Article18. Aberg KM, Radek KA, Choi EH, Kim DK, Demerjian M, Hupe M, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007. 117:3339–3349.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pseudoceramide-Containing Physiological Lipid Mixture Reduces Adverse Effects of Topical Steroids
- Comparison of Efficacy of Steroid Oint with Different Potency in Phimosis
- The Clinical Efficacy of a Multi-Lamellar Emulsion Containing Pseudoceramide in Childhood Atopic Dermatitis: An Open Crossover Study
- A Survey of Physician’s Awareness Regarding the Prescription and Side Effects of Topical Steroids
- Two Cases of Lipoatrophy after Local Corticosteroid Injection