Korean J Hematol.
2010 Sep;45(3):152-157. 10.5045/kjh.2010.45.3.152.
The how's and why's of evidence based plasma therapy
- Affiliations
-
- 1The Institute for Transfusion Medicine, Department of Pathology, University of Pittsburgh, Pittsburgh, PA, USA. myazer@itxm.org
- KMID: 2252053
- DOI: http://doi.org/10.5045/kjh.2010.45.3.152
Abstract
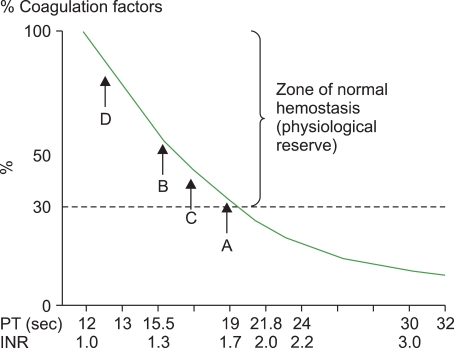
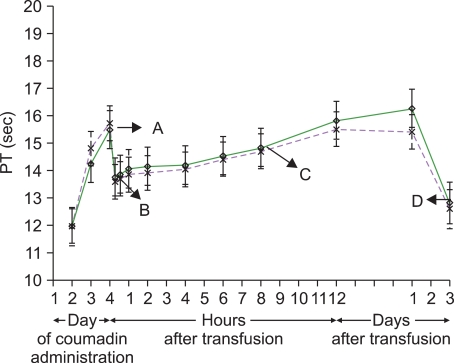
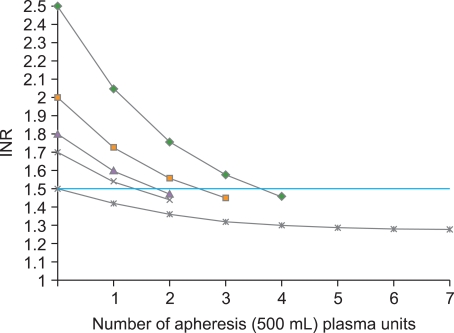
- Although traditionally fresh frozen plasma (FFP) has been the product of choice for reversing a significant coagulopathy, the modern blood bank will have several different plasma preparations which should all be equally efficacious in reversing a significant coagulopathy or arresting coagulopathic bleeding. Emerging evidence suggests that for a stable patient, transfusing plasma for an INR< or =1.5 does not confer a hemostatic benefit while unnecessarily exposing the patient to the risks associated with plasma transfusion. This review will discuss the various plasma products that are available and present some of the current literature on the clinical uses of plasma.
Keyword
Figure
Cited by 1 articles
-
Complications following an unnecessary peri-operative plasma transfusion and literature review
Jay S. Raval, Jonathan H. Waters, Darrell J. Triulzi, Mark H. Yazer
Korean J Hematol. 2012;47(4):298-301. doi: 10.5045/kjh.2012.47.4.298.
Reference
-
1. AABB. ABC. ARC. Circular of information for the use of human blood and blood components. 2002. Bethesda, MD: AABB Press.2. US Department of Health and Human Services. The 2007 national blood collection and utilization survey. 2009. Washington, DC: DHHS.3. Brecher ME. Technical Manual. 2008. 16th ed. Bethesda, MD: AABB.4. Holland LL, Foster TM, Marlar RA, Brooks JP. Fresh frozen plasma is ineffective for correcting minimally elevated international normalized ratios. Transfusion. 2005; 45:1234–1235. PMID: 15987373.
Article5. Boström F, Sjödahl M, Wehlin L, Egberg N, Lundahl J. Coagulation parameters in apheresis and leukodepleted whole-blood plasma during storage. Transfusion. 2007; 47:460–463. PMID: 17319826.
Article6. Sidhu RS, Le T, Brimhall B, Thompson H. Study of coagulation factor activities in apheresed thawed fresh frozen plasma at 1-6 degrees C for five days. J Clin Apher. 2006; 21:224–226. PMID: 16607628.7. Kakaiya RM, Morse EE, Panek S. Labile coagulation factors in thawed fresh frozen plasma prepared by two methods. Vox Sang. 1984; 46:44–46. PMID: 6702140.
Article8. Downes KA, Wilson E, Yomtovian R, Sarode R. Serial measurement of clotting factors in thawed plasma stored for 5 days. Transfusion. 2001; 41:570. PMID: 11316912.
Article9. Scott EA, Puca KE, Pietz BC, Duchateau BK, Friedman KD. Comparison and stability of ADAMTS13 activity in therapeutic plasma products. Transfusion. 2007; 47:120–125. PMID: 17207240.
Article10. von Heymann C, Keller MK, Spies C, et al. Activity of clotting factors in fresh-frozen plasma during storage at 4 degrees C over 6 days. Transfusion. 2009; 49:913–920. PMID: 19159416.11. Triulzi DJ, Kleinman S, Kakaiya RM, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009; 49:1825–1835. PMID: 19453983.
Article12. Thibault L, Beauséjour A, de Grandmont MJ, Lemieux R, Leblanc JF. Characterization of blood components prepared from whole-blood donations after a 24-hour hold with the platelet-rich plasma method. Transfusion. 2006; 46:1292–1299. PMID: 16934062.
Article13. O'Neill EM, Rowley J, Hansson-Wicher M, McCarter S, Ragno G, Valeri CR. Effect of 24-hour whole-blood storage on plasma clotting factors. Transfusion. 1999; 39:488–491. PMID: 10335998.14. Smith JF, Ness PM, Moroff G, Luban NL. Retention of coagulation factors in plasma frozen after extended holding at 1-6 degrees C. Vox Sang. 2000; 78:28–30. PMID: 10729808.15. Cardigan R, Lawrie AS, Mackie IJ, Williamson LM. The quality of fresh-frozen plasma produced from whole blood stored at 4 degrees C overnight. Transfusion. 2005; 45:1342–1348. PMID: 16078924.16. Yazer MH, Cortese-Hassett A, Triulzi DJ. Coagulation factor levels in plasma frozen within 24 hours of phlebotomy over 5 days of storage at 1 to 6 degrees C. Transfusion. 2008; 48:2525–2530. PMID: 18774964.17. Scott E, Puca K, Heraly J, Gottschall J, Friedman K. Evaluation and comparison of coagulation factor activity in fresh-frozen plasma and 24-hour plasma at thaw and after 120 hours of 1 to 6 degrees C storage. Transfusion. 2009; 49:1584–1591.18. Alhumaidan H, Cheves T, Holme S, Sweeney J. Stability of coagulation factors in plasma prepared after a 24-hour room temperature hold. Transfusion. 2010; 50:1934–1942. PMID: 20412526.
Article19. Hambleton J, Wages D, Radu-Radulescu L, et al. Pharmacokinetic study of FFP photochemically treated with amotosalen (S-59) and UV light compared to FFP in healthy volunteers anticoagulated with warfarin. Transfusion. 2002; 42:1302–1307. PMID: 12423514.
Article20. Wallis JP, Dzik S. Is fresh frozen plasma overtransfused in the United States? Transfusion. 2004; 44:1674–1675. PMID: 15504175.
Article21. Phan HH, Wisner DH. Should we increase the ratio of plasma/platelets to red blood cells in massive transfusion: what is the evidence? Vox Sang. 2010; 98:395–402. PMID: 20432517.
Article22. Segal JB, Dzik WH. Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005; 45:1413–1425. PMID: 16131373.
Article23. Dzik WH. Component therapy before bedside procedures. 2005. 2nd ed. Baltimore, MD: AABB Press.24. Stanworth SJ, Brunskill SJ, Hyde CJ, McClelland DB, Murphy MF. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol. 2004; 126:139–152. PMID: 15198745.
Article25. Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion. 2006; 46:1279–1285. PMID: 16934060.
Article26. Cheng CK, Sadek I. Fresh-frozen plasma transfusion in patients with mild coagulation abnormalities at a large Canadian transfusion center. Transfusion. 2007; 47:748. PMID: 17381635.
Article27. Youssef WI, Salazar F, Dasarathy S, Beddow T, Mullen KD. Role of fresh frozen plasma infusion in correction of coagulopathy of chronic liver disease: a dual phase study. Am J Gastroenterol. 2003; 98:1391–1394. PMID: 12818286.
Article28. Holland LL, Brooks JP. Toward rational fresh frozen plasma transfusion: the effect of plasma transfusion on coagulation test results. Am J Clin Pathol. 2006; 126:133–139. PMID: 16753596.29. Chowdhury P, Saayman AG, Paulus U, Findlay GP, Collins PW. Efficacy of standard dose and 30 mL/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol. 2004; 125:69–73. PMID: 15015971.