J Clin Neurol.
2015 Apr;11(2):164-171. 10.3988/jcn.2015.11.2.164.
Granulocyte Colony-Stimulating Factor for Amyotrophic Lateral Sclerosis: A Randomized, Double-Blind, Placebo-Controlled Study of Iranian Patients
- Affiliations
-
- 1Iranian Center of Neurological Research, Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran, Iran. harirchn@hotmail.com
- 2Neurology Department, Tehran Shariati Hospital, University of Medical Sciences, Tehran, Iran.
- 3Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
- 4Department of Immunology, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
- KMID: 2242661
- DOI: http://doi.org/10.3988/jcn.2015.11.2.164
Abstract
- BACKGROUND AND PURPOSE
The aim of this study was to determine the efficacy and tolerability of granulocyte colony-stimulating factor (G-CSF) in subjects with amyotrophic lateral sclerosis (ALS).
METHODS
Forty subjects with ALS were randomly assigned to two groups, which received either subcutaneous G-CSF (5 microg/kg/q12h) or placebo for 5 days. The subjects were then followed up for 3 months using the ALS Functional Rating Scale-Revised (ALSFRS-R), manual muscle testing, ALS Assessment Questionnaire-40, and nerve conduction studies. CD34+/CD133+ cell count and monocyte chemoattractant protein-1 (MCP-1) levels were evaluated at baseline.
RESULTS
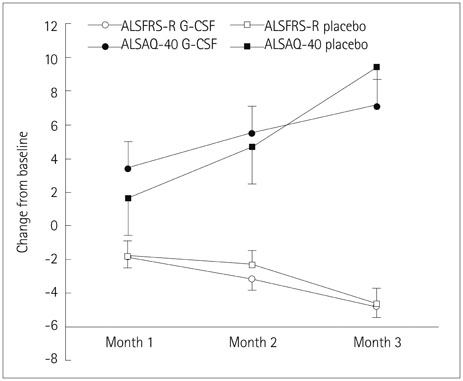
The rate of disease progression did not differ significantly between the two groups. The reduction in ALSFRS-R scores was greater in female subjects in the G-CSF group than in their counterparts in the placebo group. There was a trend toward a positive correlation between baseline CSF MCP-1 levels and the change in ALSFRS-R scores in both groups (Spearman's rho=0.370, p=0.070).
CONCLUSIONS
With the protocol implemented in this study, G-CSF is not a promising option for the treatment of ALS. Furthermore, it may accelerate disease progression in females.
Keyword
MeSH Terms
Figure
Reference
-
1. Tarella C, Rutella S, Gualandi F, Melazzini M, Scimè R, Petrini M, et al. Consistent bone marrow-derived cell mobilization following repeated short courses of granulocyte-colony-stimulating factor in patients with amyotrophic lateral sclerosis: results from a multicenter prospective trial. Cytotherapy. 2010; 12:50–59.
Article2. Zangiacomi V, Balon N, Maddens S, Lapierre V, Tiberghien P, Schlichter R, et al. Cord blood-derived neurons are originated from CD133+/CD34 stem/progenitor cells in a cell-to-cell contact dependent manner. Stem Cells Dev. 2008; 17:1005–1016.
Article3. Solaroglu I, Jadhav V, Zhang JH. Neuroprotective effect of granulocyte-colony stimulating factor. Front Biosci. 2007; 12:712–724.
Article4. Lu CZ, Xiao BG. Neuroprotection of G-CSF in cerebral ischemia. Front Biosci. 2007; 12:2869–2875.
Article5. Pollari E, Savchenko E, Jaronen M, Kanninen K, Malm T, Wojciechowski S, et al. Granulocyte colony stimulating factor attenuates inflammation in a mouse model of amyotrophic lateral sclerosis. J Neuroinflammation. 2011; 8:74.
Article6. Henriques A, Pitzer C, Dittgen T, Klugmann M, Dupuis L, Schneider A. CNS-targeted viral delivery of G-CSF in an animal model for ALS: improved efficacy and preservation of the neuromuscular unit. Mol Ther. 2011; 19:284–292.
Article7. Henriques A, Pitzer C, Dupuis L, Schneider A. G-CSF protects motoneurons against axotomy-induced apoptotic death in neonatal mice. BMC Neurosci. 2010; 11:25.
Article8. Tanaka M, Kikuchi H, Ishizu T, Minohara M, Osoegawa M, Motomura K, et al. Intrathecal upregulation of granulocyte colony stimulating factor and its neuroprotective actions on motor neurons in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2006; 65:816–825.
Article9. Zhang Y, Wang L, Fu Y, Song H, Zhao H, Deng M, et al. Preliminary investigation of effect of granulocyte colony stimulating factor on amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009; 10:430–431.
Article10. Pitzer C, Krüger C, Plaas C, Kirsch F, Dittgen T, Müller R, et al. Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis. Brain. 2008; 131(Pt 12):3335–3347.
Article11. Chiò A, Mora G, La Bella V, Caponnetto C, Mancardi G, Sabatelli M, et al. Repeated courses of granulocyte colony-stimulating factor in amyotrophic lateral sclerosis: clinical and biological results from a prospective multicenter study. Muscle Nerve. 2011; 43:189–195.
Article12. Duning T, Schiffbauer H, Warnecke T, Mohammadi S, Floel A, Kolpatzik K, et al. G-CSF prevents the progression of structural disintegration of white matter tracts in amyotrophic lateral sclerosis: a pilot trial. PLoS One. 2011; 6:e17770.
Article13. Nefussy B, Artamonov I, Deutsch V, Naparstek E, Nagler A, Drory VE. Recombinant human granulocyte-colony stimulating factor administration for treating amyotrophic lateral sclerosis: a pilot study. Amyotroph Lateral Scler. 2010; 11:187–193.
Article14. Franchignoni F, Mora G, Giordano A, Volanti P, Chiò A. Evidence of multidimensionality in the ALSFRS-R Scale: a critical appraisal on its measurement properties using Rasch analysis. J Neurol Neurosurg Psychiatry. 2013; 84:1340–1345.
Article15. Jenkinson C, Fitzpatrick R, Brennan C, Bromberg M, Swash M. Development and validation of a short measure of health status for individuals with amyotrophic lateral sclerosis/motor neurone disease: the ALSAQ-40. J Neurol. 1999; 246:Suppl 3. III16–III21.16. Shamshiri H, Eshraghian MR, Ameli N, Nafissi S. Validation of the Persian version of the 40-item amyotrophic lateral sclerosis assessment questionnaire. Iran J Neurol. 2013; 12:102–105.17. Great Lakes ALS Study Group. A comparison of muscle strength testing techniques in amyotrophic lateral sclerosis. Neurology. 2003; 61:1503–1507.18. Wilms H, Sievers J, Dengler R, Bufler J, Deuschl G, Lucius R. Intrathecal synthesis of monocyte chemoattractant protein-1 (MCP-1) in amyotrophic lateral sclerosis: further evidence for microglial activation in neurodegeneration. J Neuroimmunol. 2003; 144:139–142.
Article19. Zhao LR, Navalitloha Y, Singhal S, Mehta J, Piao CS, Guo WP, et al. Hematopoietic growth factors pass through the blood-brain barrier in intact rats. Exp Neurol. 2007; 204:569–573.
Article20. Martino M, Callea I, Condemi A, Dattola A, Irrera G, Marcuccio D, et al. Predictive factors that affect the mobilization of CD34(+) cells in healthy donors treated with recombinant granulocyte colony-stimulating factor (G-CSF). J Clin Apher. 2006; 21:169–175.
Article21. Chiò A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, et al. Prognostic factors in ALS: A critical review. Amyotroph Lateral Scler. 2009; 10:310–323.
Article22. Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010; 411:1570–1579.
Article23. Henkel JS, Engelhardt JI, Siklós L, Simpson EP, Kim SH, Pan T, et al. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004; 55:221–235.
Article24. Cronin S, Hardiman O, Traynor BJ. Ethnic variation in the incidence of ALS: a systematic review. Neurology. 2007; 68:1002–1007.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sweet Syndrome in a Child with Aplastic Anemia after Receiving Recombinant Granulocyte Colony-stimulating Factor
- Two cases of congenital agranulocytosis treated with recombinant human granulocyte colony-stimulating factor
- The effect of granulocyte colony-stimulating factor in chemotherapy of acute myelogenous leukemia
- The effects on the production of platelet activating factor in the cultured human endothelial cells by interleukin-6 and granulocyte macrophage colony stimulating factor
- Effect of combination gene therapy with herpes simplex virus thymidine kinase suicidal gene and granulocyte-macrophage colony-stimulating factor gene in murine melanoma model