Korean J Lab Med.
2006 Jun;26(3):185-191. 10.3343/kjlm.2006.26.3.185.
Development of Enzyme Linked Immunosorbent Assay for Erythropoietin
- Affiliations
-
- 1Department of Bioscience and Biotechology, Konkuk University, Seoul, Korea.
- 2Department of Laboratory Medicine, Dankook University College of Medicine, Cheon-An, Korea.
- 3College of Pharmacy, Chungbuk University, Cheong-Ju, Korea. dcmoon@chungbuk.ac.kr
- KMID: 2238885
- DOI: http://doi.org/10.3343/kjlm.2006.26.3.185
Abstract
-
BACKGROUND: The aim of our study was to optimize and establish erythropoietin (EPO) enzyme linked immunosorbent assay (ELISA) system.
METHODS
We prepared several monoclonal and polyclonal antibodies specific to human-EPO. The best combinations of antibodies for coating and detecting antibodies were selected for the establishment of ELISA. We tested several methods such as a competitive EIA and a sandwich ELISA.
RESULTS
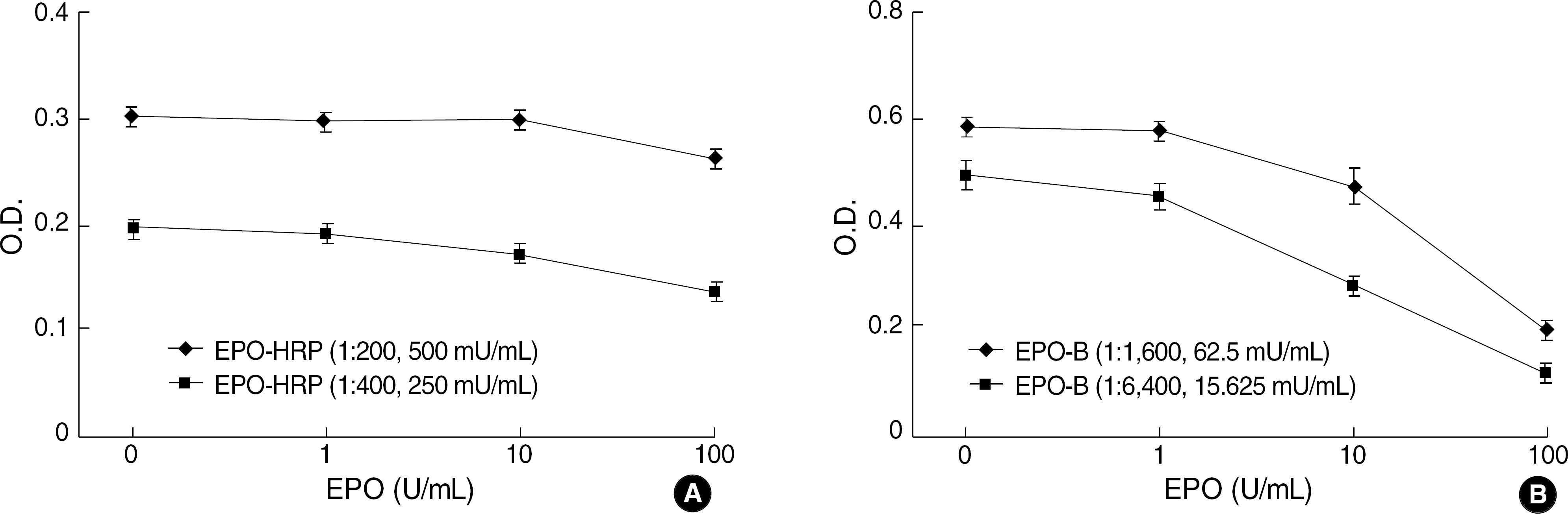
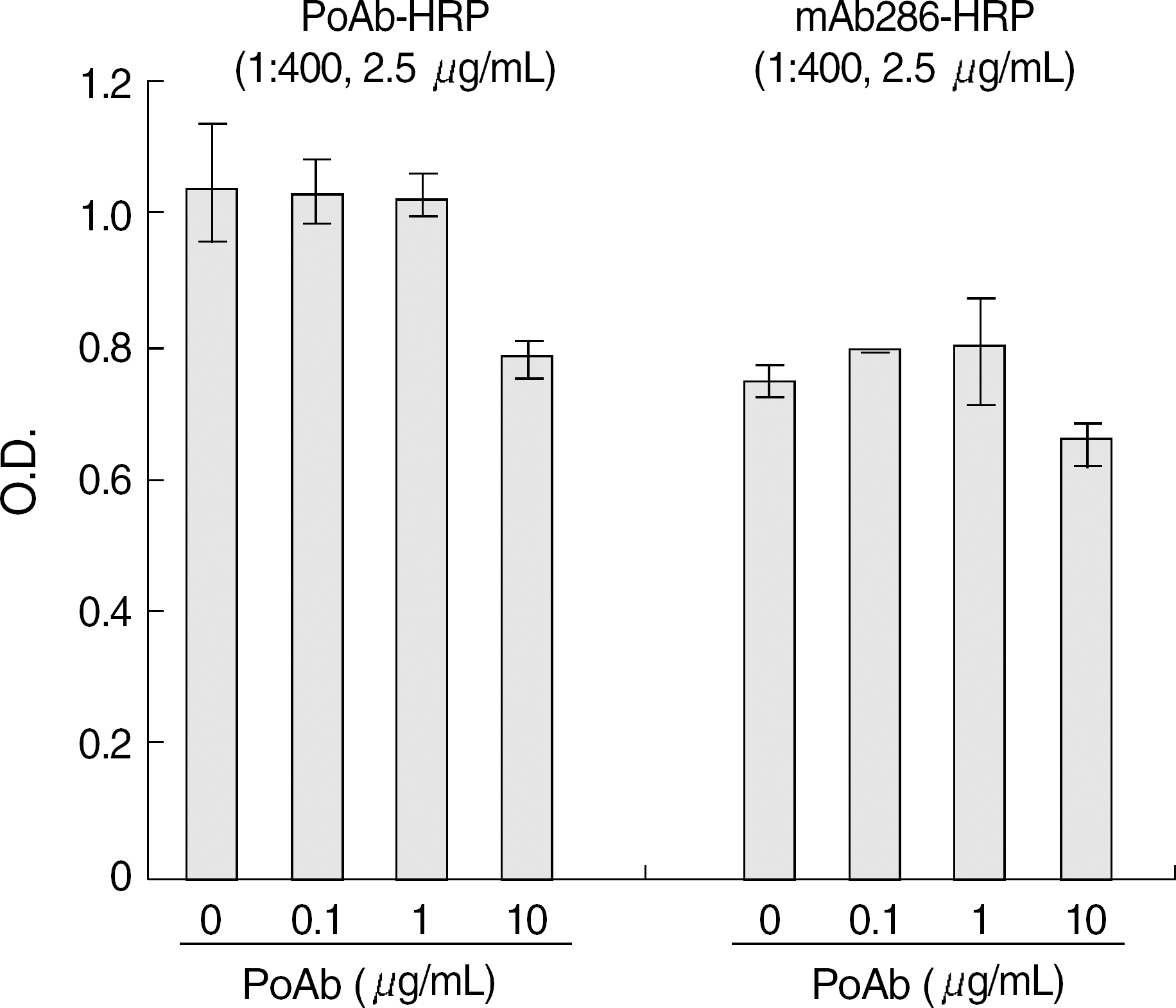
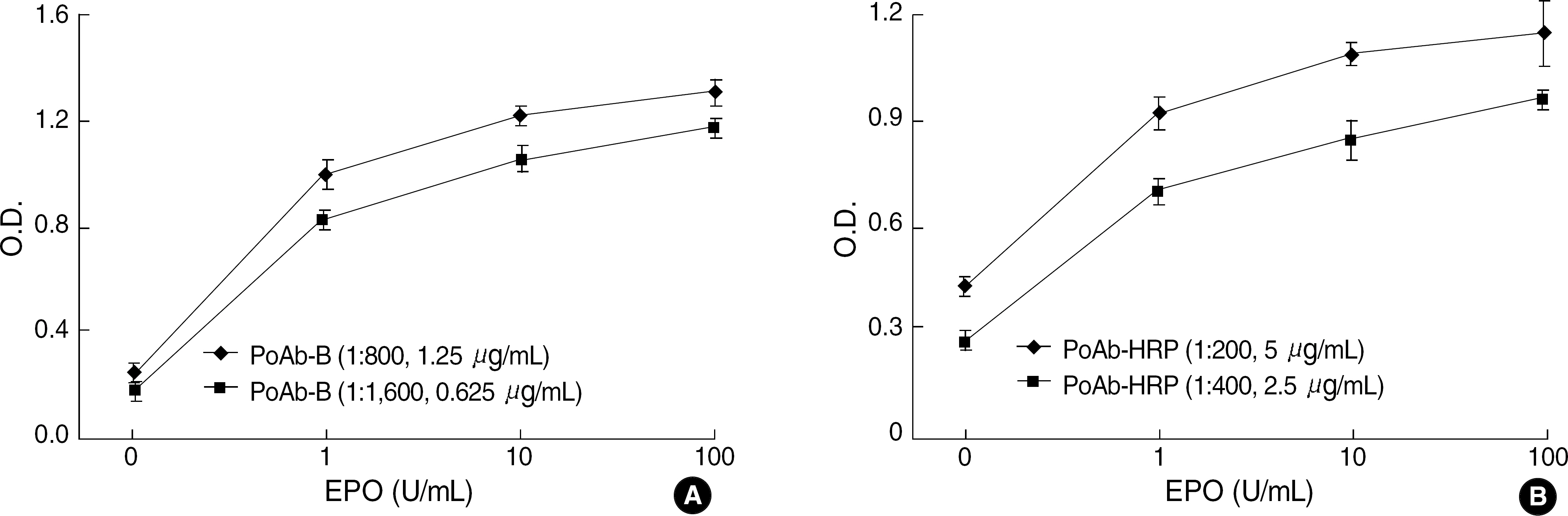
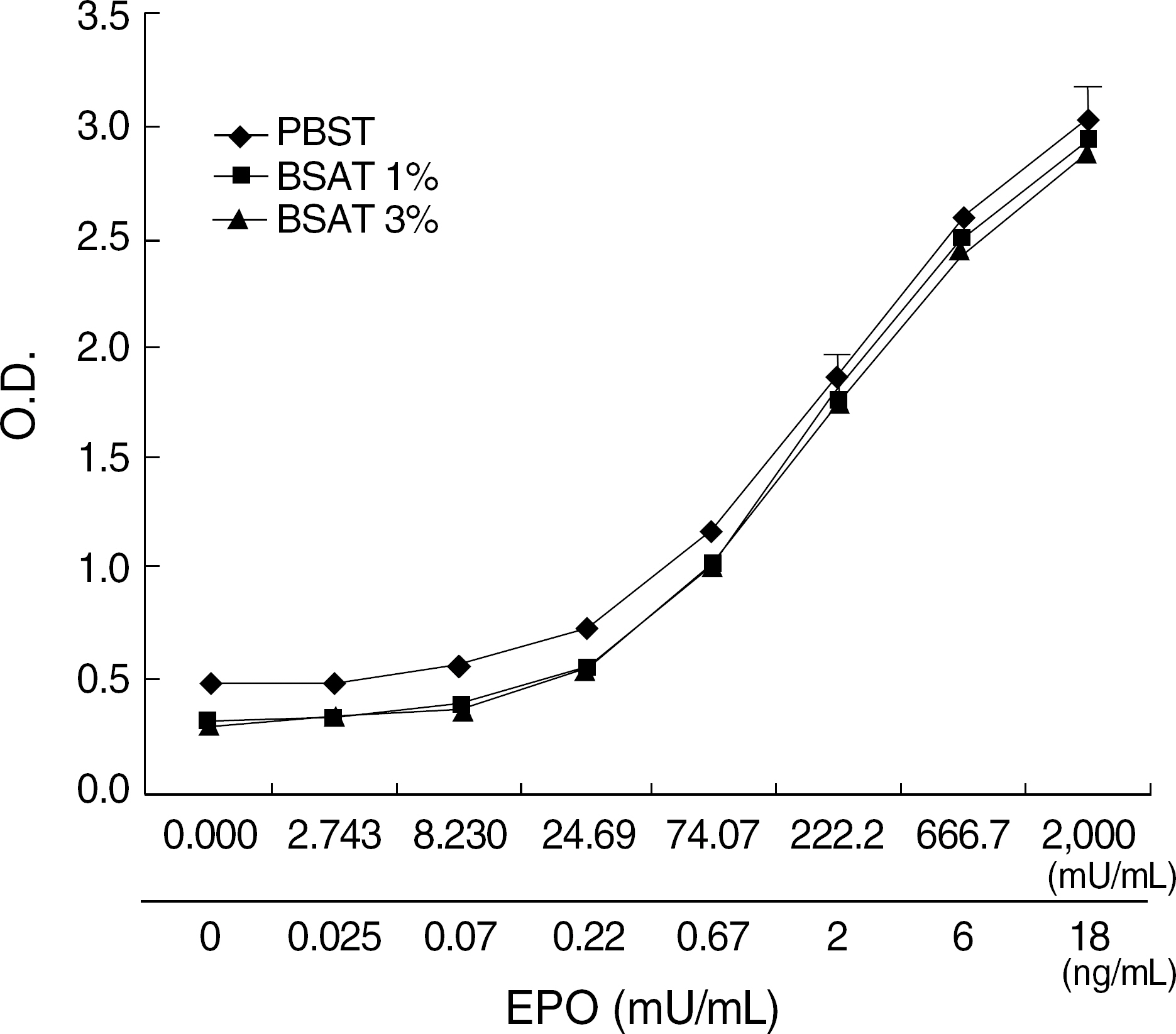
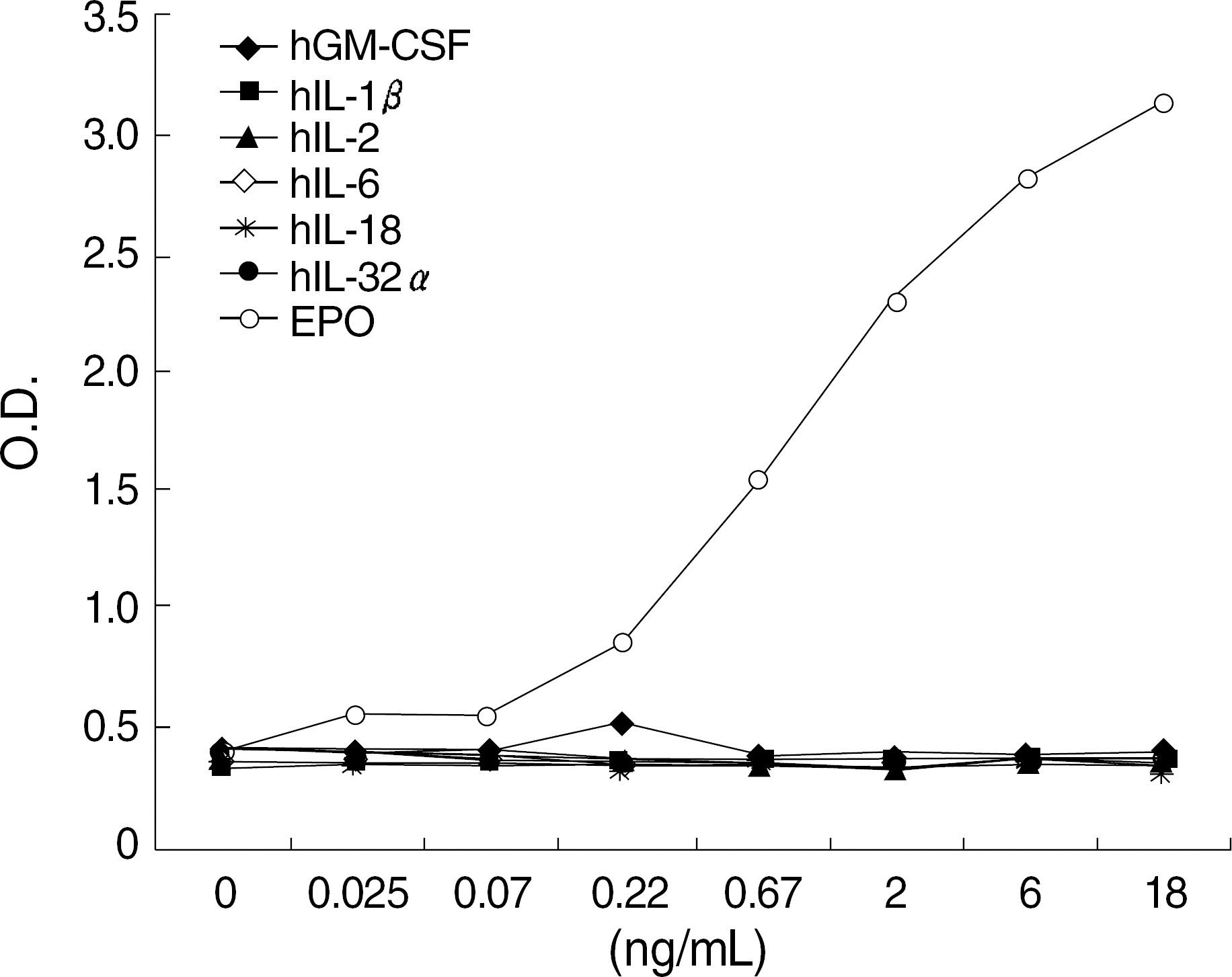
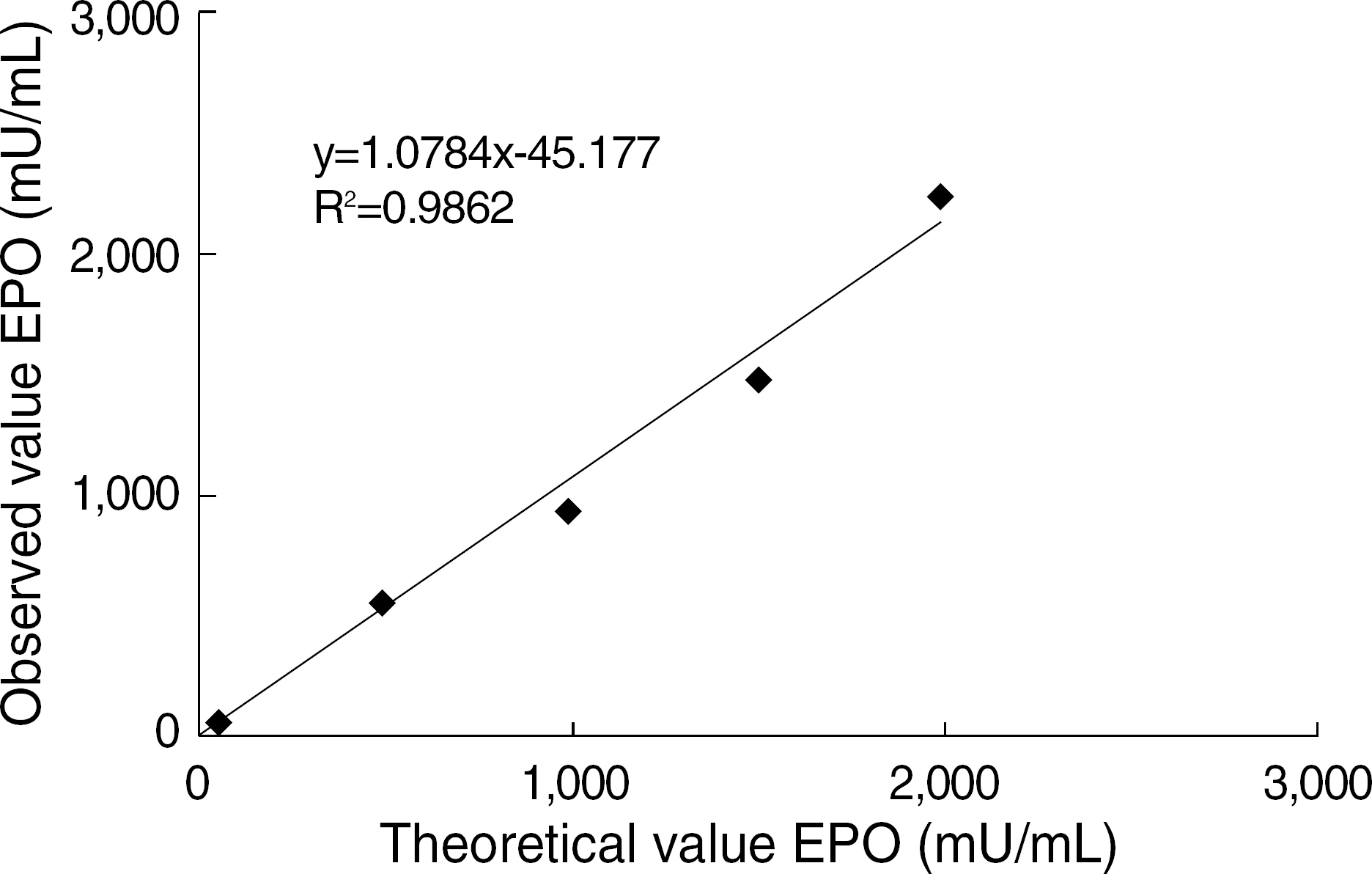
The best sandwich ELISA was optimized compared to competitive EIA when purified polyclonal antibody (PoAb) was used as a coating antibody and biotinylated PoAb as a detecting antibody. This sandwich ELISA easily detected EPO when PoAb pairs were used compared to the ELISA using monoclonal antibody and PoAb. There were no significant differences between the effects of various blocking solutions on the performance of sandwich ELISA using biotinylated antibody. The ELISA system using PBST containing 3% BSA as a blocking solution can sensitively detect EPO (10 mU/mL) in a broad range of EPO concentrations (10-2,000 mU/mL) and there were cross-reactions with other cytokines).
CONCLUSIONS
EPO can be easily determined by using biotinylated PoAb as a detecting antibody and another PoAb as a coating antibody.
Keyword
Figure
Reference
-
References
1. Abu-Qare AW, Abou-Donia MB. High performance liquid chromatographic determination of diazinon, permethrin, DEET (N, N-diethyl-m-toluamide), and their metabolites in rat plasma and urine. Fresenius. J Anal Chem. 2001; 70:403–7.2. Chamkasem N, Hill KD, Sewell GW. High-performance liquid chromatographic column-switching technique for the determination of intermediates of anaerobic degradation of toluene in ground water microcosm. J Chromatogr. 1991; 587:185–91.
Article3. Cass QB, Oliveira RV, De Pietro AC. Determination of gossypol enantiomer ratio in cotton plants by chiral higher-performance liquid chromatography. J Agric Food Chem. 2004; 52:5822–7.
Article4. Klvanova J, Brtko J. Selected retinoids: determination by isocratic normal-phase HPLC. Endocr Regul. 2002; 36:133–41.5. Cousino MA, Jarbawi TB, Halsall HB, Heineman WR. Pushing down the limits of detection: molecular needles in a haystack. Anal Chem. 1997; 69:544–9A.6. Chen SF, Xu Y, Ip MP. Electrochemical enzyme immunoassay for serum prostate-specific antigen at low concentrations. Clin Chem. 1997; 43:1459–61.
Article7. Messina GA, Torriero AA, De Vito IE, Olsina RA, Raba J. Continuous-flow/stopped-flow system using an immunobiosensor for quantification of human serum immunoglobulin G (IgG) antibodies to Helicobacter pylori. Anal Biochem. 2005; 337:195–202.8. Qu Y, Berghman LR, Vandesande F. An electrochemical enzyme immunoassay for chicken luteinizing hormone: extension of the detection limit by adequate control of the nonspecific adsorption. Anal Biochem. 1998; 259:167–75.
Article9. Wang X, Chen F, Wan PJ, Huang G. Development of monoclonal antibody-based enzyme-linked immunosorbent assay for gossypol analysis in cottonseed meals. J Agric Food Chem. 2004; 52:7793–7.
Article10. Zola H. Monoclonal antibodies: Production, purification, analysis, quality control, storage, and distribution. In. Zola H, editor. Monoclonal antibodies: A manual of techniques. Boca Raton: CRC Press, FL;1987. p. 70–6.11. Wang X, Plhak LC. Monoclonal antibodies for the analysis of gossypol in cottonseed products. J Agric Food Chem. 2004; 52:709–12.
Article12. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227:680–5.
Article13. Conkerton EJ, Frampton VL. Reaction of gossypol with free epsilon-amino groups of lysine in proteins. Arch Biochem Biophys. 1959; 81:130–4.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of anti-borrelia burgdorferi antibody by enzyme-linked immunosorbent assay in Korea
- Evaluation of enzymum system@(ES-300) for enzyme linked immunosorbent assay: comparison with RIA and CLIA for T3, T4, fT4 and TSH
- Evaluation of enzymum system@(ES-300) for enzyme linked immunosorbent assay: comparison with RIA and CLIA for T3, T4, fT4 and TSH
- A Case of Scrotal Sparganosis Detected by Enzyme Linked Immunosorbent Assay (ELISA)
- Comparison of enzyme-linked immunosorbent assay, indirect immunofluorescent antibody test and indirect immunoperoxidase antibody test in setecting antibodies to rickettsia tsutsugamushi