Clin Orthop Surg.
2015 Sep;7(3):344-350. 10.4055/cios.2015.7.3.344.
Factors Affecting Survival in Patients Undergoing Palliative Spine Surgery for Metastatic Lung and Hepatocellular Cancer: Dose the Type of Surgery Influence the Surgical Results for Metastatic Spine Disease?
- Affiliations
-
- 1Department of Orthopedic Surgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. boscoa@catholic.ac.kr
- KMID: 2234089
- DOI: http://doi.org/10.4055/cios.2015.7.3.344
Abstract
- BACKGROUND
Surgical treatment for metastatic spine disease has been becoming more prominent with the help of technological advances and a few favorable reports on the surgery. In cases of this peculiar condition, it is necessary to establish the role of surgery and analyze the factors affecting survival.
METHODS
From January 2011 to April 2015, 119 patients were surgically treated for metastatic spine lesions. To reduce the bias along the heterogeneous cancers, the primary cancer was confined to either the lung (n = 25) or the liver (n = 18). Forty-three patients (male, 32; female, 11; mean age, 57.5 years) who had undergone palliative surgery were enrolled in this study. Posterior decompression and fusion was performed in 30 patients (P group), and anteroposterior (AP) reconstruction was performed in 13 patients (AP group) for palliative surgery. Pre- and postoperative (3 months) pain (visual analogue scale, VAS), performance status (Karnofsky performance score), neurologic status (American Spinal Injury Association [ASIA] grade), and spinal instability neoplastic score (SINS) were compared. The survival period and related hazard factors were also assessed by Kaplan-Meier and Cox regression analysis.
RESULTS
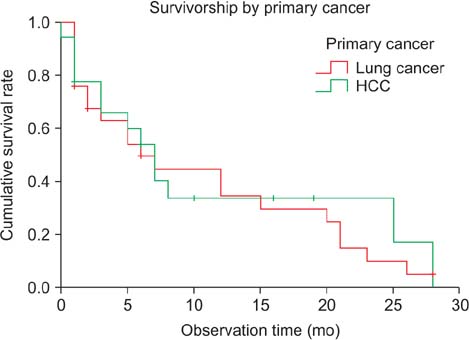
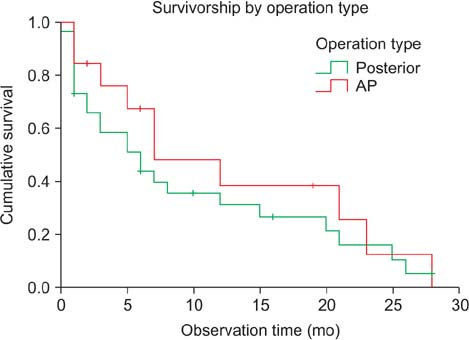
Most patients experienced improvements in pain and performance status (12.3% +/- 17.2%) at 3 months postoperatively. In terms of neurologic recovery, 9 patients (20.9%) graded ASIA D experienced neurological improvement to ASIA E while the remainder was status quo. In an analysis according to operation type, there was no significant difference in patient demographics. At 12 months postoperatively, cumulative survival rates were 31.5% and 38.7% for the P group and the AP group, respectively (p > 0.05). Survival was not affected by the pre- and postoperative pain scale, Tokuhashi score, neurologic status, SINS, or operation type. Preoperative Karnofsky performance score (hazard ratio, 0.93; 95% confidence interval [CI], 0.89 to 0.96) and improvement of performance status after surgery (hazard ratio, 0.95; 95% CI, 0.92 to 0.97) significantly affected survival after operation.
CONCLUSIONS
There was no significant difference in surgical outcomes and survival rates between posterior and AP surgery for metastatic lesions resulting from lung and hepatocellular cancer. Preoperative Karnofsky score and improvement of performance status had a significant impact on the survival rate following surgical treatment for these metastatic spine lesions.
Keyword
MeSH Terms
-
Aged
Back Pain
*Decompression, Surgical/adverse effects/methods/mortality
Female
Humans
Kaplan-Meier Estimate
Liver Neoplasms/*pathology
Lung Neoplasms/*pathology
Male
Middle Aged
Pain, Intractable
Palliative Care/*methods
Prognosis
Retrospective Studies
*Spinal Fusion/adverse effects/methods/mortality
*Spinal Neoplasms/mortality/surgery
Spine/*surgery
Figure
Reference
-
1. Daffner SD. Spinal tumors. In : Fynn JM, editor. American Academy of Orthopaedic Surgeons. OKU 10: orthopaedic knowledge update. Rosemont: American Academy of Orthopaedic Surgeon;2011. p. 553–564.2. Lewandrowski KU, Anderson ME, McLain RF. Tumors of the spine. In : Herkowitz HN, Garfin SR, Eismont FJ, Bell GR, Balderston RA, editors. Rothman-Simeone the spine. 6th ed. Philadelphia, PA: Elsevier Sounders;2011. p. 1480–1512.3. Gardocki RJ, Camillo FX. Tumors of the spine. In : Canale ST, Beaty JH, editors. Campbell's operative orthopaedics. 12th ed. St. Louis: Elsevier/Mosby;2013. p. 2033–2050.4. Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the Cancer Care Ontario Practice Guidelines Initiative's Neuro-Oncology Disease Site Group. J Clin Oncol. 2005; 23(9):2028–2037.
Article5. Loblaw DA, Mitera G, Ford M, Laperriere NJ. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int J Radiat Oncol Biol Phys. 2012; 84(2):312–317.
Article6. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005; 366(9486):643–648.
Article7. Falicov A, Fisher CG, Sparkes J, Boyd MC, Wing PC, Dvorak MF. Impact of surgical intervention on quality of life in patients with spinal metastases. Spine (Phila Pa 1976). 2006; 31(24):2849–2856.
Article8. Ibrahim A, Crockard A, Antonietti P, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine. 2008; 8(3):271–278.
Article9. Li H, Gasbarrini A, Cappuccio M, et al. Outcome of excisional surgeries for the patients with spinal metastases. Eur Spine J. 2009; 18(10):1423–1430.
Article10. Quan GM, Vital JM, Aurouer N, et al. Surgery improves pain, function and quality of life in patients with spinal metastases: a prospective study on 118 patients. Eur Spine J. 2011; 20(11):1970–1978.
Article11. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 2005; 30(19):2186–2191.
Article12. Putz C, Wiedenhofer B, Gerner HJ, Furstenberg CH. Tokuhashi prognosis score: an important tool in prediction of the neurological outcome in metastatic spinal cord compression. A retrospective clinical study. Spine (Phila Pa 1976). 2008; 33(24):2669–2674.
Article13. Tokuhashi Y, Ajiro Y, Umezawa N. Outcome of treatment for spinal metastases using scoring system for preoperative evaluation of prognosis. Spine (Phila Pa 1976). 2009; 34(1):69–73.
Article14. Papastefanou S, Alpantaki K, Akra G, Katonis P. Predictive value of Tokuhashi and Tomita scores in patients with metastatic spine disease. Acta Orthop Traumatol Turc. 2012; 46(1):50–56.
Article15. Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976). 2010; 35(22):E1221–E1229.16. Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011; 29(22):3072–3077.
Article17. Kim HJ, Buchowski JM, Moussallem CD, Rose PS. Modern techniques in the treatment of patients with metastatic spine disease. Instr Course Lect. 2013; 62:375–382.
Article18. Molina CA, Gokaslan ZL, Sciubba DM. Diagnosis and management of metastatic cervical spine tumors. Orthop Clin North Am. 2012; 43(1):75–87.
Article19. Feiz-Erfan I, Fox BD, Nader R, et al. Surgical treatment of sacral metastases: indications and results. J Neurosurg Spine. 2012; 17(4):285–291.
Article20. Wiedenhofer B, Mohlenbruch M, Hemmer S, Lehner B, Klockner K, Akbar M. Vertebral stability in management of spinal metastases: criteria and strategies for operative interventions. Orthopade. 2012; 41(8):623–631.21. Horn L, Pao W, Johnson DH. Neoplasms of the lung. In : Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison's principles of internal medicine. 18th ed. New York: McGraw-Hill;2012. p. 737–753.22. Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In : MacLeod CM, editor. Evaluation of chemotherapeutic agents. New York: Columbia University Press;1949. p. 196–197.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preliminary study of anesthetic risk factors in surgery for pathologic fractures secondary to metastatic tumors
- Metastatic Spinal Tumor
- A Clinical Analysis of Metastatic Spine Tumors: Analysis of Prognostic Factors and Scoring System for Prognostic Evaluation
- Outcomes after Surgery for Spinal Metastasis of Colorectal Origin: Case Series
- Unstable Cervical Spine Caused by Metastatic Tumors