Korean J Gastroenterol.
2014 Jun;63(6):378-381. 10.4166/kjg.2014.63.6.378.
Clinical Review and Case Report of Ceftriaxone-associated Gallbladder Pseudolithiasis in Adult
- Affiliations
-
- 1Department of Surgery, Yeungnam University College of Medicine, Daegu, Korea. dslee@med.yu.ac.kr
- KMID: 2234041
- DOI: http://doi.org/10.4166/kjg.2014.63.6.378
Abstract
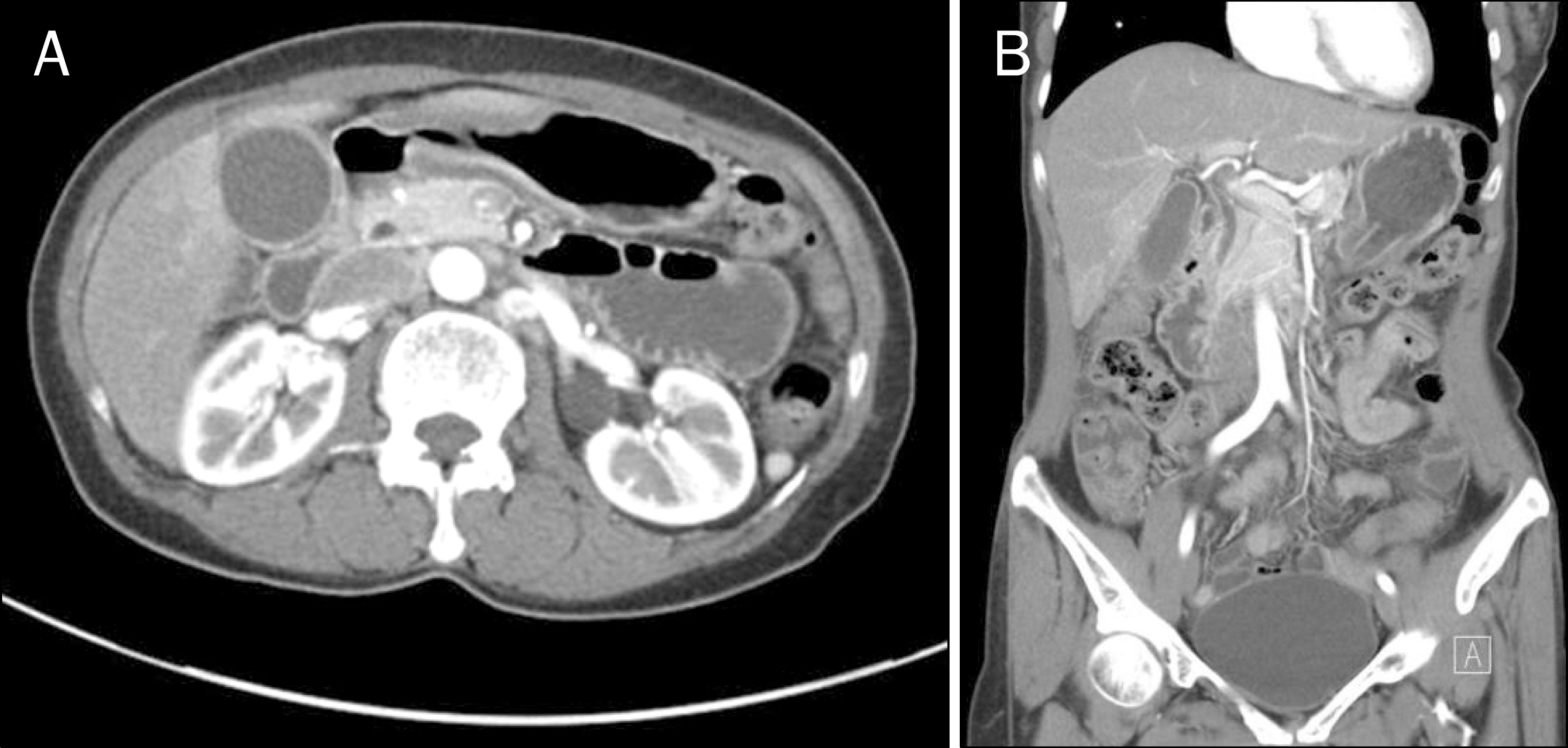
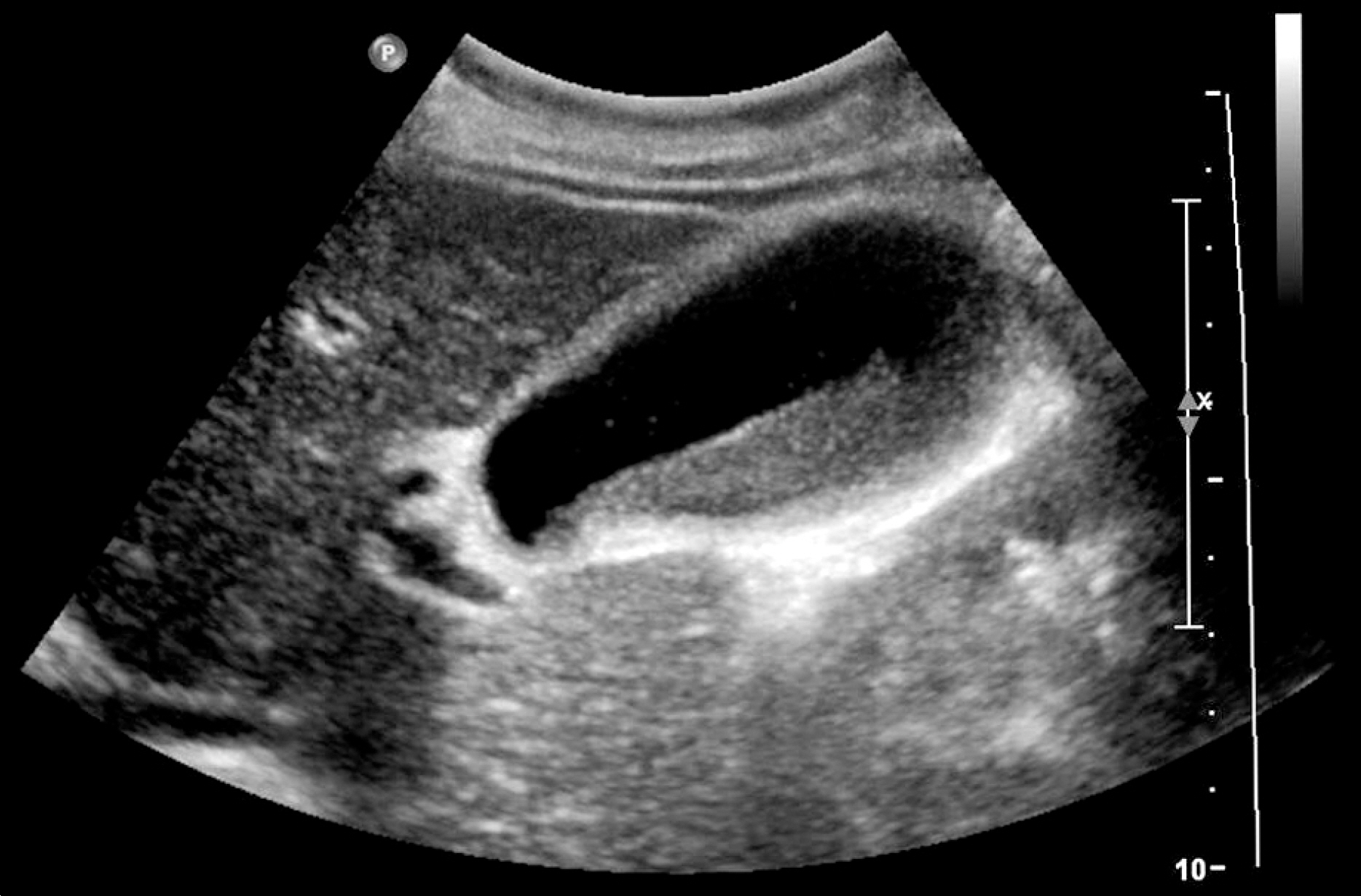
- Although ceftriaxone can be used safely in most instances, it can sometimes induce biliary sludge or stone formation. Most of the patients remain asymptomatic and children are more susceptible to develop this condition, but adults can be affected as well. Because sludge or stones disappear after discontinuing ceftriaxone, this condition is referred to as ceftriaxone-associated pseudolithiasis. A 54-year-old woman was admitted to a local clinic for management of ileus. During admission, she had received ceftriaxone and metronidazole, and had been on nil per os for the past 6 days. She was then referred to our hospital for cholecystectomy due to persistent right upper quadrant pain. Although imaging studies showed gallbladder sludge, pseudolithiasis was suspected because of ceftriaxone administration history and prolonged fasting. After careful watch-and-wait, the condition resolved spontaneously after ceftriaxone discontinuation. Our clear understanding on ceftriaxone-associated gallbladder pseudolithiasis allowed us to avoid an unnecessary cholecystectomy. Herein, we report the case of a 54-year-old woman with ceftriaxone-associated gallbladder pseudolithiasis that was successfully managed by ceftriaxone discontinuation alone.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Schaad UB, Wedgwood-Krucko J, Tschaeppeler H. Reversible ceftriaxone-associated biliary pseudolithiasis in children. Lancet. 1988; 2:1411–1413.
Article2. Heim-Duthoy KL, Caperton EM, Pollock R, Matzke GR, Enthoven D, Peterson PK. Apparent biliary pseudolithiasis during ceftriaxone therapy. Antimicrob Agents Chemother. 1990; 34:1146–1149.3. Schaad UB, Tschäppeler H, Lentze MJ. Transient formation of precipitations in the gallbladder associated with ceftriaxone therapy. Pediatr Infect Dis. 1986; 5:708–710.
Article4. Shiffman ML, Keith FB, Moore EW. Pathogenesis of ceftriaxone-associated biliary sludge. In vitro studies of calcium-cef-triaxone binding and solubility. Gastroenterology. 1990; 99:1772–1778.
Article5. Pigrau C, Pahissa A, Gropper S, Sureda D, Martinez Vazquez JM. Ceftriaxone-associated biliary pseudolithiasis in adults. Lancet. 1989; 2:165.
Article6. Choi YY, Jung YH, Choi SM, Lee CS, Kim D, Hur KY. Gallbladder pseudolithiasis caused by ceftriaxone in young adult. J Korean Surg Soc. 2011; 81:423–426.
Article7. Seo JH, Hwang JM, Park SS. Antituberculosis medication as a possible epigenetic factor of Leber's hereditary optic neuropathy. Clin Experiment Ophthalmol. 2010; 38:363–366.
Article8. Park HZ, Lee SP, Schy AL. Ceftriaxone-associated gallbladder sludge. Identification of calcium-ceftriaxone salt as a major component of gallbladder precipitate. Gastroenterology. 1991; 100:1665–1670.
Article9. Rivera M, García-Herrera AL, Burguera V, Sosa-Barrios H, Palomares JR, Quereda C. Ceftriaxone-associated gallbladder pseudolithiasis in a PD patient. Perit Dial Int. 2011; 31:97–99.
Article10. Papadopoulou F, Efremidis S, Karyda S, et al. Incidence of cef-triaxone-associated gallbladder pseudolithiasis. Acta Paediatr. 1999; 88:1352–1355.11. Kim S, Gura KM, Puder M. Acute necrotizing cholecystitis: a rare complication of ceftriaxone-associated pseudolithiasis. Pediatr Surg Int. 2006; 22:562–564.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ceftriaxone-Associated Biliary Pseuodolithiasis: Sonographic and CT Findings
- A Case of Reversible Biliary Pseudolithiasis After Ceftriaxone Therapy
- Sonographic Findings of Ceftriaxone-associated Gallbladder Pseudolithiasis
- Biliary Pseudolithiasis in Children: To Avoid Unnecessary Surgical Procedure
- Ceftriaxone Associated Biliary Pseudolithiasis