Korean Circ J.
2009 Aug;39(8):335-339. 10.4070/kcj.2009.39.8.335.
A Case of Fabry Cardiomyopathy
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Seoul Paik Hospital, Inje University Medical College, Seoul, Korea.
- 2Department of Internal Medicine, School of Medicine, Kyung Hee University, Seoul, Korea. kkabee@dreamwiz.com
- 3Department of Pediatrics, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 4Division of Cardiac Repair and Regeneration, Graduate School of Medical and Dental Sciences, agoshima University, Kagoshima, Japan.
- 5Department of Cardiovascular, Respiratory and Metabolic Medicine, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan.
- KMID: 2225684
- DOI: http://doi.org/10.4070/kcj.2009.39.8.335
Abstract
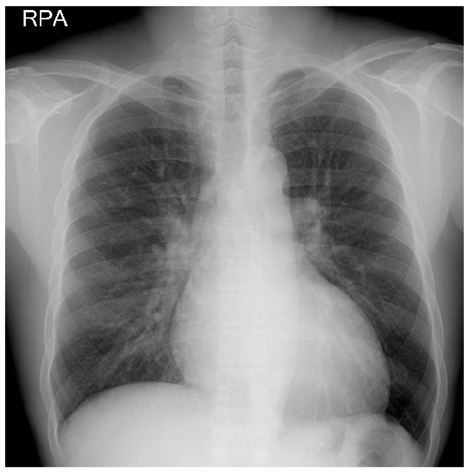
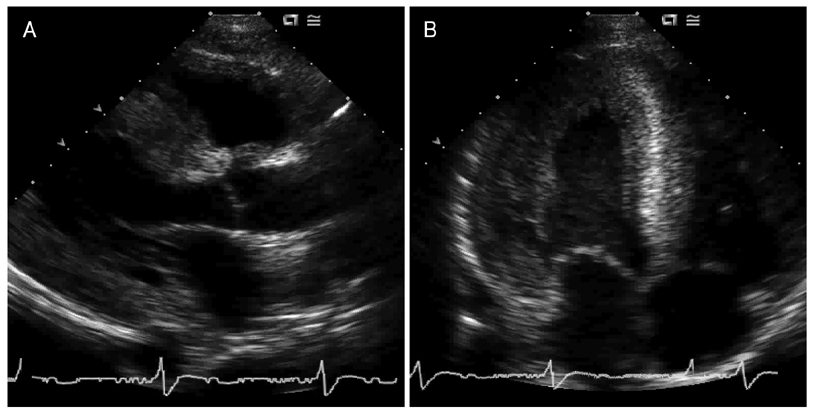
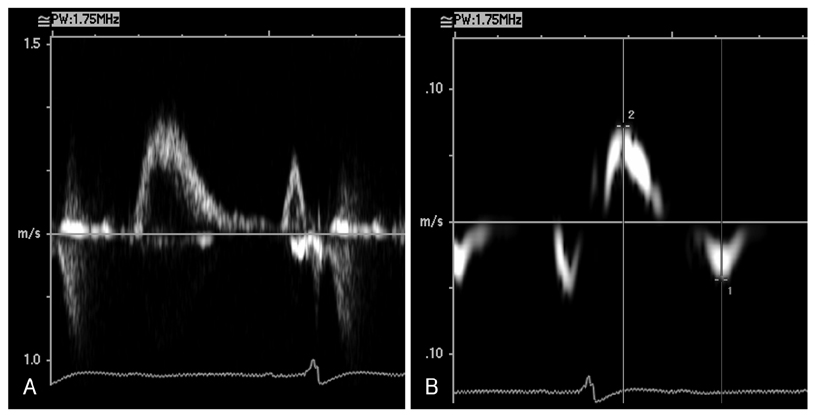
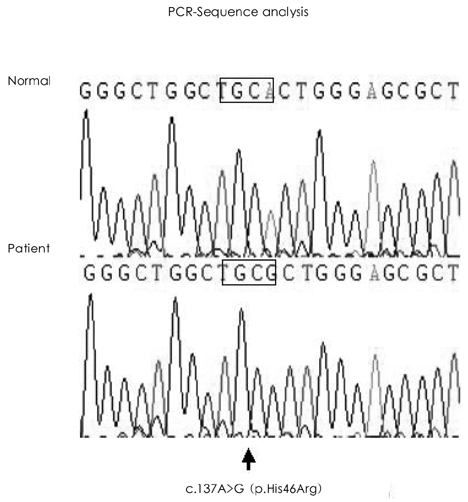
- In the absence of hypertension, hypertrophic cardiomyopathy is the most common cause of left ventricular hypertrophy (LVH). However, it has been reported that up to 3% of males with unexplained LVH have Fabry disease, an X-linked disorder of glycophospholipid metabolism that is due to a deficiency in the lysosomal enzyme alpha-galactosidase A (alpha-Gal A). A 44-year-old man was admitted to our hospital with palpitations. He had a history of chronic renal failure diagnosed at age 33 followed by kidney transplantation performed at our institution 2 years later, as well as long-standing hypohidrosis. His medications included prednisolone (5 mg daily), mycophenolate mofetil (1,000 mg, bid), and cyclosporine (150 mg, bid). On hospital day two, an echocardiogram demonstrated increased left ventricular wall thickness (septal wall thickness of 28 mm, posterior wall thickness of 20 mm). Diastolic dysfunction was noted on transmitral flow patterns and tissue Doppler imaging. The patient was found to have low plasma alpha-Gal A activity. A previously reported H46R missense mutation was detected in his alpha-Gal A gene and the patient was subsequently diagnosed with Fabry disease.
MeSH Terms
-
Adult
alpha-Galactosidase
Cardiomyopathies
Cardiomyopathy, Hypertrophic
Cyclosporine
Fabry Disease
Genes, vif
Humans
Hypertension
Hypertrophy, Left Ventricular
Hypohidrosis
Kidney Failure, Chronic
Kidney Transplantation
Male
Mutation, Missense
Mycophenolic Acid
Plasma
Prednisolone
Cyclosporine
Mycophenolic Acid
Prednisolone
alpha-Galactosidase
Figure
Reference
-
1. Desnick RJ, Ioannou YA, Eng CM. Scriver CR, Beaudet AL, Sly WS, editors. α-Galactosidase A deficiency: Fabry disease. The Metabolic and Molecular Bases of Inherited Disease. 2001. New York: McGraw-Hill;3733–3774.2. Tanaka H, Adachi K, Yamashita Y, Toshima H, Koga Y. Four cases of Fabry's disease mimicking hypertrophic cardiomyopathy. J Cardiol. 1988. 18:705–718.3. Jeong JW. Hypertrophic cardiomyopathy. Korean Circ J. 2002. 32:7–14.4. Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human α-galactosidase A: replacement therapy in Fabry's disease. N Engl J Med. 2001. 345:9–16.5. Weidemann F, Niemann M, Breunig F, et al. Long-term effects of enzyme replacement therapy on Fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation. 2009. 119:524–529.6. Burrow TA, Hopkin RJ, Leslie ND, Tinkle BT, Grabowski GA. Enzyme reconstitution/replacement therapy for lysosomal storage disease. Curr Opin Pediatr. 2007. 19:628–635.7. Sachdev B, Takenaka T, Teraguchi H, et al. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation. 2002. 105:1407–1411.8. Chimenti C, Pieroni M, Morgante E, et al. Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation. 2004. 110:1047–1053.9. von Scheidt W, Eng CM, Fitzmaurice TF, et al. An atypical variant of Fabry's disease with manifestations confined to the myocardium. N Engl J Med. 1991. 324:395–399.10. Nakao S, Takenaka T, Maeda M, et al. An atypical variant of Fabry's disease in men with left ventricular hypertrophy. N Engl J Med. 1995. 333:288–293.11. Cohen IS, Fluri-Lundeen J, Wharton TP. Two dimensional echocardiographic similarity of Fabry's disease to cardiac amyloidosis: a function of ultrastructural analogy? J Clin Ultrasound. 1983. 11:437–441.12. Linhart A, Palecek T, Bultas J, et al. New insights in cardiac structural changes in patients with Fabry's disease. Am Heart J. 2000. 139:1101–1108.13. Kawano M, Takenaka T, Otsuji Y, et al. Significance of asymmetric basal posterior wall thinning in patients with cardiac Fabry's disease. Am J Cardiol. 2007. 99:261–263.14. Takenaka T, Teraguchi H, Yoshida A, et al. Terminal stage cardiac findings in patients with cardiac Fabry disease: an electrocardiographic, echocardiographic, and autopsy study. J Cardiol. 2008. 51:50–59.15. Shah JS, Hughes DA, Sachdev B, et al. Prevalence and clinical significance of cardiac arrhythmia in Anderson-Fabry Disease. Am J Cardiol. 2005. 96:842–846.16. Jastrzebski M, Bacior B, Dimitrow PP, Kawecka-Jaszcz K. Electrophysiological study in a patient with Fabry disease and a short PQ interval. Europace. 2006. 8:1045–1047.17. Hariharan S. Recurrent and de novo diseases after renal transplantation. Semin Dial. 2000. 13:195–199.18. Obrador GT, Ojo A, Thadhani R. End-stage renal disease in patients with Fabry disease. J Am Soc Nephrol. 2002. 13:Suppl 2. S144–S146.19. Schiffmann R, Kopp JB, Austin HA 3rd, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001. 285:2743–2749.20. Banikazemi M, Bultas J, Waldek S, et al. Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med. 2007. 146:77–86.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fabry Disease Presenting with Hypertrophic Cardiomyopathy and Tricuspid Regurgitation
- Fabry Disease that Phenocopies Hypertrophic Cardiomyopathy: a thorough Genetic ‘Detective’ Identifies the ‘Rogue’ Hidden in the GLA Gene
- Fabry Disease in a Family: Four Patients and Five Carriers
- A Case of Cerebral Aneurysmal Subarachnoid Hemorrhage in Fabry's Disease
- Fabry's disease: a case report and review of literatures reported in Korea