Korean Circ J.
2010 Dec;40(12):671-676. 10.4070/kcj.2010.40.12.671.
A Case of Cardiac Amyloidosis With Diuretic-Refractory Pleural Effusions Treated With Bevacizumab
- Affiliations
-
- 1Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea. kimyd@mail.donga.ac.kr
- 2Department of Radiology, Dong-A University College of Medicine, Busan, Korea.
- 3Department of Pathology, Dong-A University College of Medicine, Busan, Korea.
- KMID: 2225167
- DOI: http://doi.org/10.4070/kcj.2010.40.12.671
Abstract
- Cardiac amyloidosis describes a clinical disorder caused by infiltration of abnormal insoluble fibrils in the heart, characterized by progressive heart failure and a grave prognosis. Pleural effusion in cardiac amyloidosis may represent a sign of heart failure, but it can also result from pleural infiltration of amyloid, manifested by recurrent large fluid accumulations. Recently, the role of vascular endothelial growth factor (VEGF) has been implicated in the pathogenesis of refractory pleural effusion. We report a case of a 53 year-old female patient with cardiac amyloidosis who presented with recurrent accumulation of large pleural effusions. She was initially treated with high dose loop diuretics, but the pleural effusion persisted, with the daily amount of drainage averaging 1 L/day. Accumulation of pleural fluid did not subside after 3 cycles of melphalan/prednisolone therapy. After the introduction of bevacizumab, an anti-VEGF antibody, the amount of pleural effusion decreased significantly. Efficacy of anti-VEGF therapy for refractory pleural effusions needs to be defined through further studies.
Keyword
MeSH Terms
-
Amyloid
Amyloidosis
Antibodies, Monoclonal, Humanized
Drainage
Female
Heart
Heart Diseases
Heart Failure
Humans
Pleural Effusion
Prognosis
Sodium Potassium Chloride Symporter Inhibitors
Vascular Endothelial Growth Factor A
Bevacizumab
Amyloid
Antibodies, Monoclonal, Humanized
Sodium Potassium Chloride Symporter Inhibitors
Vascular Endothelial Growth Factor A
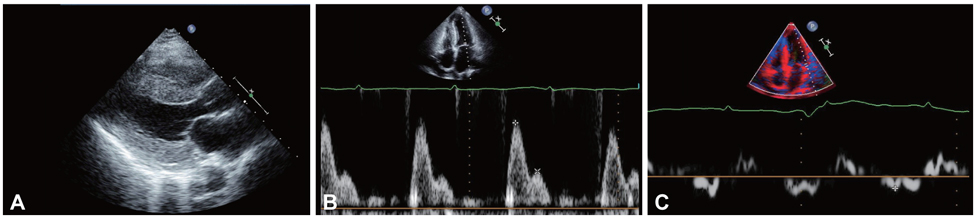
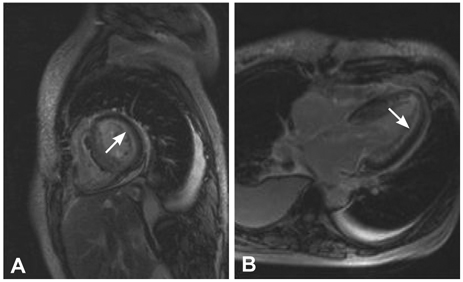
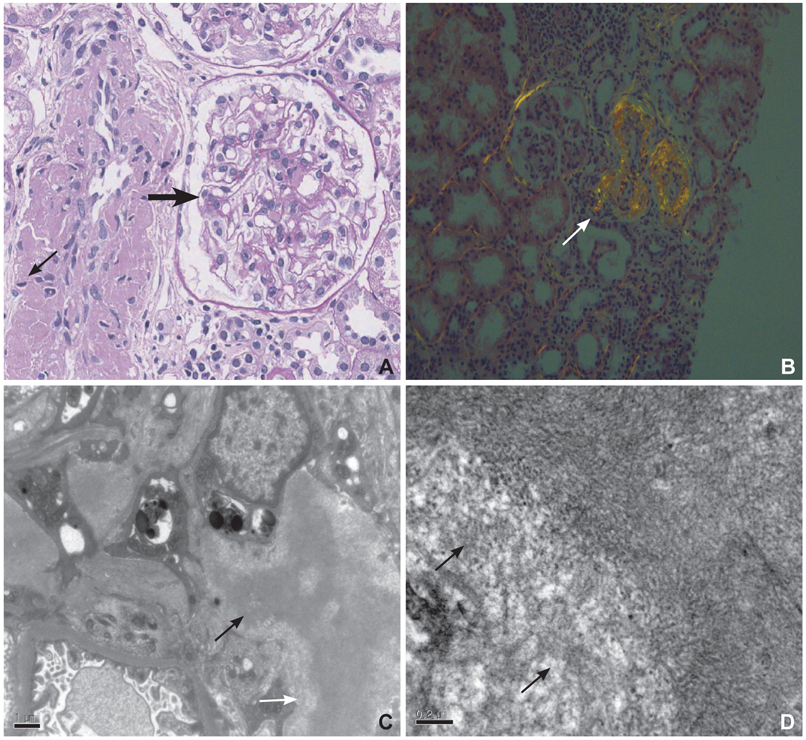
Figure
Reference
-
1. Selvanayagam JB, Hawkins PN, Paul B, Myerson SG, Neubauer S. Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol. 2007. 50:2101–2110.2. Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005. 112:2047–2060.3. Kim YJ, Choi SO, Kim MK, et al. A case of familial cardiac amyloidosis. Korean Circ J. 2004. 34:520–526.4. Berk JL. Pleural effusions in systemic amyloidosis. Curr Opin Pulm Med. 2005. 11:324–328.5. Grove CS, Lee YC. Vascular endothelial growth factor: the key mediator in pleural effusion formation. Curr Opin Pulm Med. 2002. 8:294–301.6. Sack U, Hoffmann M, Zhao XJ, et al. Vascular endothelial growth factor in pleural effusions of different origin. Eur Respir J. 2005. 25:600–604.7. Pichelmayer O, Zielinski C, Raderer M. Response of a nonmalignant pleural effusion to bevacizumab. N Engl J Med. 2005. 353:740–741.8. Hoyer RJ, Leung N, Witzig TE, Lacy MQ. Treatment of diuretic refractory pleural effusions with bevacizumab in four patients with primary systemic amyloidosis. Am J Hematol. 2007. 82:409–413.9. Vogelsberg H, Mahrholdt H, Deluigi CC, et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis. J Am Coll Cardiol. 2008. 51:1022–1030.10. Berk JL, Keane J, Seldin DC, et al. Persistent pleural effusions in primary systemic amyloidosis. Chest. 2003. 124:969–977.11. Smith RR, Hutchins GM, Moore GW, Humphrey RL. Type and distribution of pulmonary parenchymal and vascular amyloid: correlation with cardiac amyloid. Am J Med. 1979. 66:96–104.12. Bontemps F, Tillie-Leblond I, Coppin MC, et al. Pleural amyloidosis: thoracoscopic aspects. Eur Respir J. 1995. 8:1025–1027.13. Kinasewitz GT. Transudative effusions. Eur Respir J. 1997. 10:714–718.14. Albertine KH, Wiener-Kronish JP, Staub NC. The structure of the parietal pleura and its relationship to pleural liquid dynamics in sheep. Anat Rec. 1984. 208:401–409.15. Light RW, Hamm H. Malignant pleural effusion: would the real cause please stand up? Eur Respir J. 1997. 10:1701–1702.16. Mohammed KA, Nasreen N, Hardwick J, Logie CS, Patterson CE, Antony VB. Bacterial induction of pleural mesothelial monolayer barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001. 281:L119–L125.17. Feng D, Nagy JA, Hipp J, Dvorak HF, Dvorak AM. Vesiculo-vacuolar barrier organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J Exp Med. 1996. 183:1981–1986.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on Diagnostic Value of Lysozyme Activity in Various pleural effusions
- Comparison of Characteristics of Pleural Fluid and Blood in Mycoplasmal and Tuberculous Pleural Effusions
- A study on lactic dehydrogenase activity of tuberculous pleural effusion
- Ferritin Assay in Pleural Effusion
- A Case of Systemic Amyloidosis