Korean Circ J.
2011 Mar;41(3):130-136. 10.4070/kcj.2011.41.3.130.
Effect of Hyperkalemia and Hemolysis Caused by Hyperacute Rejection on Cardiac Function in Pig to Human Ex Vivo Xenogeneic Cardiac Perfusion Model
- Affiliations
-
- 1Department of Thoracic and Cardiovascular Surgery, School of Medicine, Konkuk University, Seoul, Korea.
- 2Xenotransplantation Research Center, Seoul National University Hospital, Seoul, Korea. jrl@plaza.snu.ac.kr
- 3Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul, Korea.
- KMID: 2225126
- DOI: http://doi.org/10.4070/kcj.2011.41.3.130
Abstract
- BACKGROUND AND OBJECTIVES
Hyperacute rejection (HAR) is a major obstacle to successful xenotransplantation of vascularized organs. This study was conducted to observe the effect of hemolysis of perfused human whole blood on pig heart function, and determine the major risk factors for preservation of xenoperfused cardiac function using ex-vivo pig to human xenogeneic cardiac perfusion model.
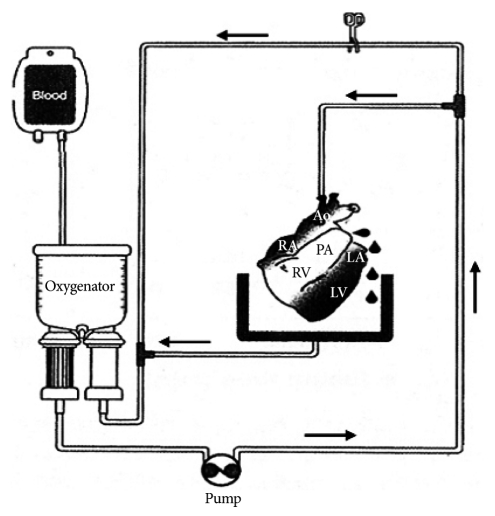
MATERIALS AND METHODS
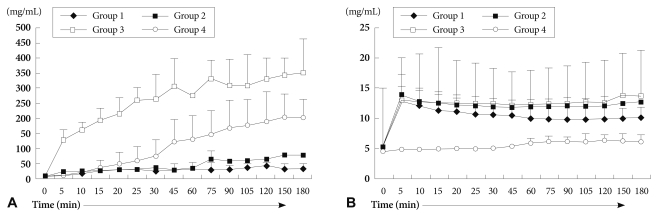
Harvested pig hearts were perfused with normal human whole blood (group 1), two different types of pre-treated human whole blood (group 2: immunoglobulins were depleted by plasmapheresis, group 3: pre-treated with plasmapheresis, GAS914, cobra venom factor (CVF) and steroid), and normal porcine whole blood as control (group 4) for 3 hours.
RESULTS
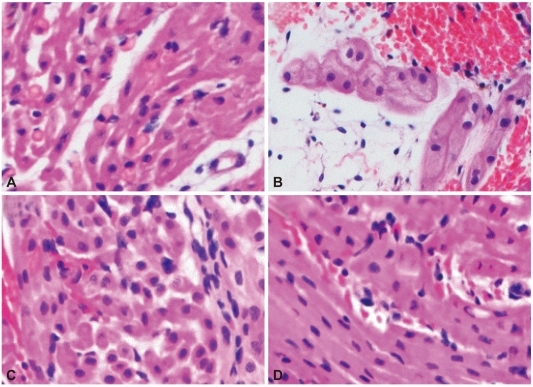
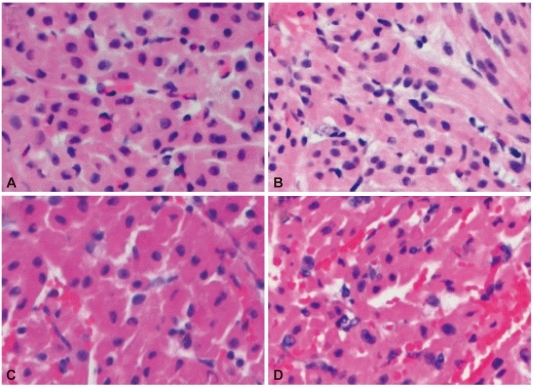
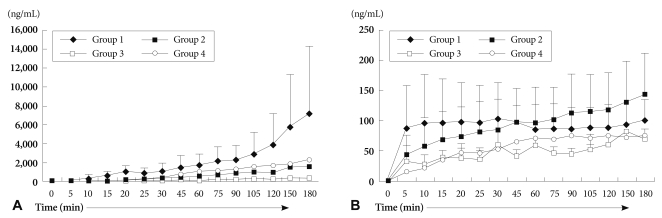
Duration of heart beat was significantly prolonged in group 2 and group 3. Histological examination showed widespread HAR features but was gradually delayed in groups 2 and 3 compared to group 1. The absolute levels of serum creatine kinase-MB and Troponin I increased gradually, and was lower in group 3. Serum hemoglobin levels were rapidly increased in groups 3 and 4, compared to group 1. Extracellular potassium level increased sharply from the beginning of blood perfusion in groups 1, 2 and 3, compared to group 4.
CONCLUSION
Pretreatment of human whole blood, including immunoglobulin depletion, CVF and steroid reduced and delayed the destruction of pig myocardium by HAR. However, the increased extracellular potassium levels in groups 1, 2 and 3 reflected that these treatments could not prohibit myocardial injury by HAR.
MeSH Terms
-
Cobra Venoms
Creatine
Diphtheria Toxoid
Extracorporeal Circulation
Haemophilus Vaccines
Heart
Hemoglobins
Hemolysis
Humans
Hyperkalemia
Immunoglobulins
Myocardium
Perfusion
Plasmapheresis
Potassium
Rejection (Psychology)
Risk Factors
Transplantation, Heterologous
Trisaccharides
Troponin I
Cobra Venoms
Creatine
Diphtheria Toxoid
Haemophilus Vaccines
Hemoglobins
Immunoglobulins
Potassium
Trisaccharides
Troponin I
Figure
Reference
-
1. Lee JR, Kim HK, Kim JS, et al. Effect of antibody titer on xenograft survival in pig-to-dog heterotropic cardiac xenotransplantation. Korean J Thorac Cardiovasc Surg. 2004; 37:391–400.2. Sachs DH. The pig as a xenograft donor. Pathol Biol (Paris). 1994; 42:217–219. PMID: 8090569.3. Sykes M, Lee LA, Sachs DH. Xenograft tolerance. Immunol Rev. 1994; 141:245–276. PMID: 7868155.4. Tonomura N, Shimizu A, Wang S, et al. Pig islet xenograft rejection in a mouse model with an established human immune system. Xenotransplantation. 2008; 15:129–135. PMID: 18447886.5. Rydberg L, Hallberg E, Bjorck S. Studies on the removal of anti-pig in the human by plasmapheresis/immunoadsorption. Xenotransplantation. 1995; 2:253–259.6. Leventhal JR, John R, Fryer JP, et al. Removal of baboon and human antiporcine IgG and IgM natural antibodies by immunoadsorption: results of in vitro and in vivo studies. Transplantation. 1995; 59:294–300. PMID: 7839454.7. Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha 1, 3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 2008; 172:1471–1481. PMID: 18467706.8. Kimikawa M, Agishi T, Teraoka S, Suga H, Hayasaka Y, Ota K. Prolongation of cardiac xenograft survival by double filtration plasmapheresis and ex vivo xenoantibody adsorption. Transplant Proc. 1992; 24:447. PMID: 1566383.9. Nishitai R, Ikai I, Matsuo K, et al. Influence of extracorporeal porcine liver perfusion on nonhuman primates: minimizing hemolysis improves subsequent survival. Liver Transpl. 2001; 7:615–622. PMID: 11460229.10. Macchiarini P, Mazmanian GM, Oriol R, et al. Ex vivo lung model of pig-to-human hyperacute rejection. J Thorac Cardiovasc Surg. 1997; 114:315–325. PMID: 9305182.11. Baek SH. The current concept of cell therapy for heart failure. Korean Circ J. 2005; 35:415–423.12. Kroshus TJ, Bolman RM, Dalmasso AP. Selective IgM depletion prolongs organ survival in an ex vivo model of pig-to-human xenotransplantation. Transplantation. 1996; 62:5–12. PMID: 8693544.13. Katopodis AG, Warner RG, Duthaler RO, et al. Removal of anti-Galα1,3Gal xenoantibodies with an injectable polymer. J Clin Invest. 2002; 110:1869–1877. PMID: 12488437.14. Tanemura M, Yin D, Chong AS, Galili U. Differential immune responses to α-gal epitopes on xenografts and allografts: implications for accommodation in xenotransplantation. J Clin Invest. 2000; 105:301–310. PMID: 10675356.15. Brandl U, Michel S, Erhardt M, et al. Administration of GAS914 in an orthotopic pig to baboon heart transplantation model. Xenotransplantation. 2005; 12:134–141. PMID: 15693844.16. Walpen AJ, Mohacsi P, Frey C, Roos A, Daha MR, Rieben R. Activation of complement pathways in xenotransplantation: an in vivo study. Transpl Immunol. 2002; 9:271–280. PMID: 12180841.17. Kim CH, Zo JH, Seo JD, Lee YW, Brooks E. Evolutional change of vasoactive substances in rat model of chronic heart failure. Korean Circ J. 1997; 27:767–773.18. Kobayashi T, Taniguchi S, Neethling FA, et al. Delayed xenograft rejection of pig-to-baboon cardiac transplants after cobra venom factor therapy. Transplantation. 1997; 64:1255–1261. PMID: 9371665.19. Vogel CW, Fritzinger DC, Hew BE, Thorne M, Bammert H. Recombinant cobra venom factor. Mol Immunol. 2004; 41:191–199. PMID: 15159065.20. Grimm H, Mages P, Lindemann G, et al. Evidence against a pivotal role of preformed antibodies in delayed rejection of a guinea pig-to-rat heart xenograft. J Thorac Cardiovasc Surg. 2000; 119:477–487. PMID: 10694606.21. Oberholzer J, Yu D, Triponez F, et al. Decomplementation with cobra venom factor prolongs survival of xenografted islets in a rat to mouse model. Immunology. 1999; 97:173–180. PMID: 10447729.22. Fischel RJ, Bolman RM 3rd, Platt JL, Najarian JS, Bach FH, Matas AJ. Removal of IgM antiendothelial antibodies results in prolonged cardiac xenograft survival. Transplant Proc. 1990; 22:1077–1078. PMID: 2190373.23. Suga H, Ishida H, Kimikawa M, et al. Prolongation of cardiac xenograft function after reduction of natural antibodies using double filtration plasmapheresis. ASAIO Trans. 1991; 37:M433–M434. PMID: 1751224.24. Sato Y, Kimikawa M, Suga H, et al. Prolongation of cardiac xenograft survival by double filtration plasmapheresis and ex vivo immunoadsorption. ASAIO J. 1992; 38:M673–M675. PMID: 1457946.25. Satoh S, Terajima H, Yagi T, et al. Humoral injury in porcine livers perfused with human whole blood. Transplantation. 1997; 64:1117–1123. PMID: 9355826.26. Yeh JH, Chen WH, Chiu HC. Complication of double-filtration plasmapheresis. Transfusion. 2004; 44:1621–1625. PMID: 15504168.27. Tambourgi DV, dos Santos MC, Furtado M de F, de Freitas MC, da Silva WD, Kipnis TL. Pro-inflammatory activities in elapsed snake venoms. Br J Pharmacol. 1994; 112:723–727. PMID: 7921595.28. Ahn JM, Kim JJ, Song MG, et al. The efficacy and safety of an immunosuppressive regimen including the use of mycophenolate mofetil and an interleukin-2 monoclonal antibody in heart transplant patients. Korean Circ J. 2006; 36:794–801.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardiac arrest during excision of a huge sacrococcygeal teratoma: A report of two cases
- The Effect of Intravenous Immunoglobulin on Hyperacute and Acclerated Rejection in Heart Transplantation of the Rat
- Interspecies incompatibility of CD200 contribute to the xenogeneic immune response
- Using CT to Evaluate Cardiac Function
- Repeated cardiac arrests caused by transfusion-related hyperkalemia and massive bleeding : A case report