Korean Circ J.
2016 Jan;46(1):56-62. 10.4070/kcj.2016.46.1.56.
Visualization of the Critical Isthmus by Tracking Delayed Potential in Edited Windows for Scar-Related Ventricular Tachycardia
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. oys@catholic.ac.kr
- 2Division of Cardiology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Division of Cardiology, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2223770
- DOI: http://doi.org/10.4070/kcj.2016.46.1.56
Abstract
- BACKGROUND AND OBJECTIVES
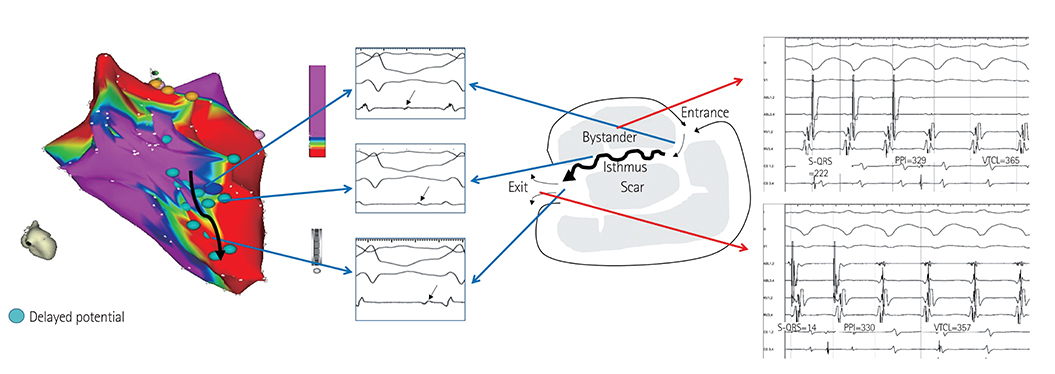
Identifying the critical isthmus of slow conduction is crucial for successful treatment of scar-related ventricular tachycardia. Current 3D mapping is not designed for tracking the critical isthmus and may lead to a risk of extensive ablation. We edited the algorithm to track the delayed potential in order to visualize the isthmus and compared the edited map with a conventional map.
SUBJECTS AND METHODS
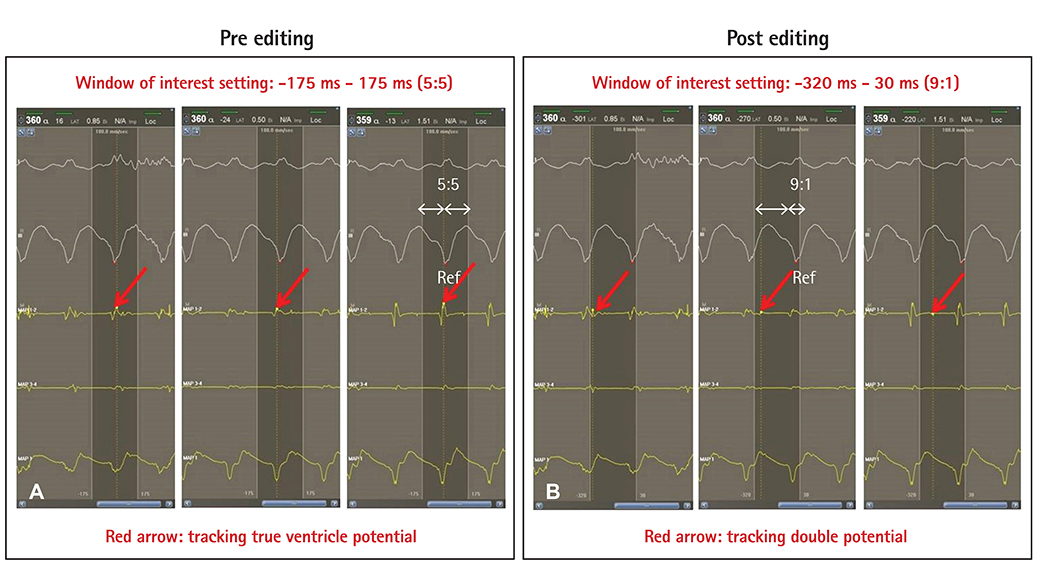
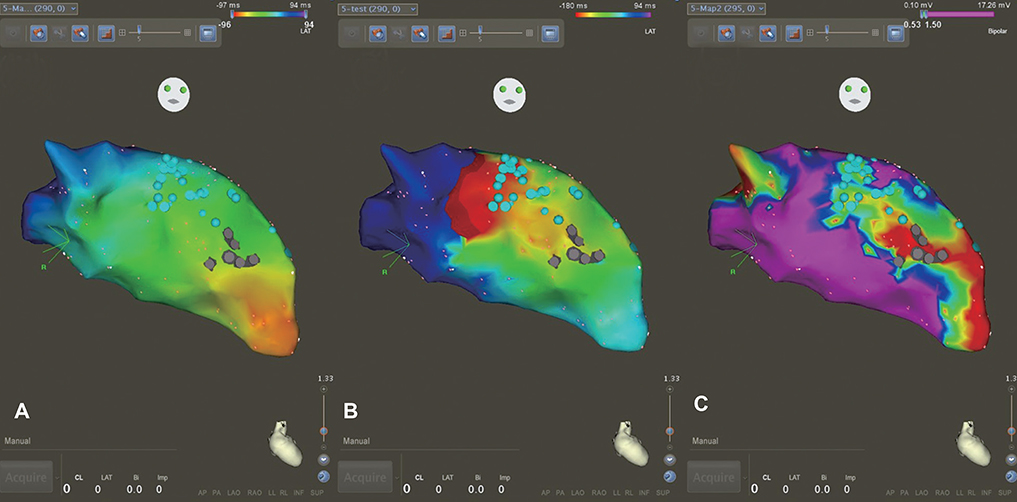
We marked every point that showed delayed potential with blue color. After substrate mapping, we edited to reset the annotation from true ventricular potential to delayed potential and then changed the window of interest from the conventional zone (early, 50-60%; late, 40-50% from peak of QRS) to the edited zone (early, 80-90%; late, 10-20%) for every blue point. Finally, we compared the propagation maps before and after editing.
RESULTS
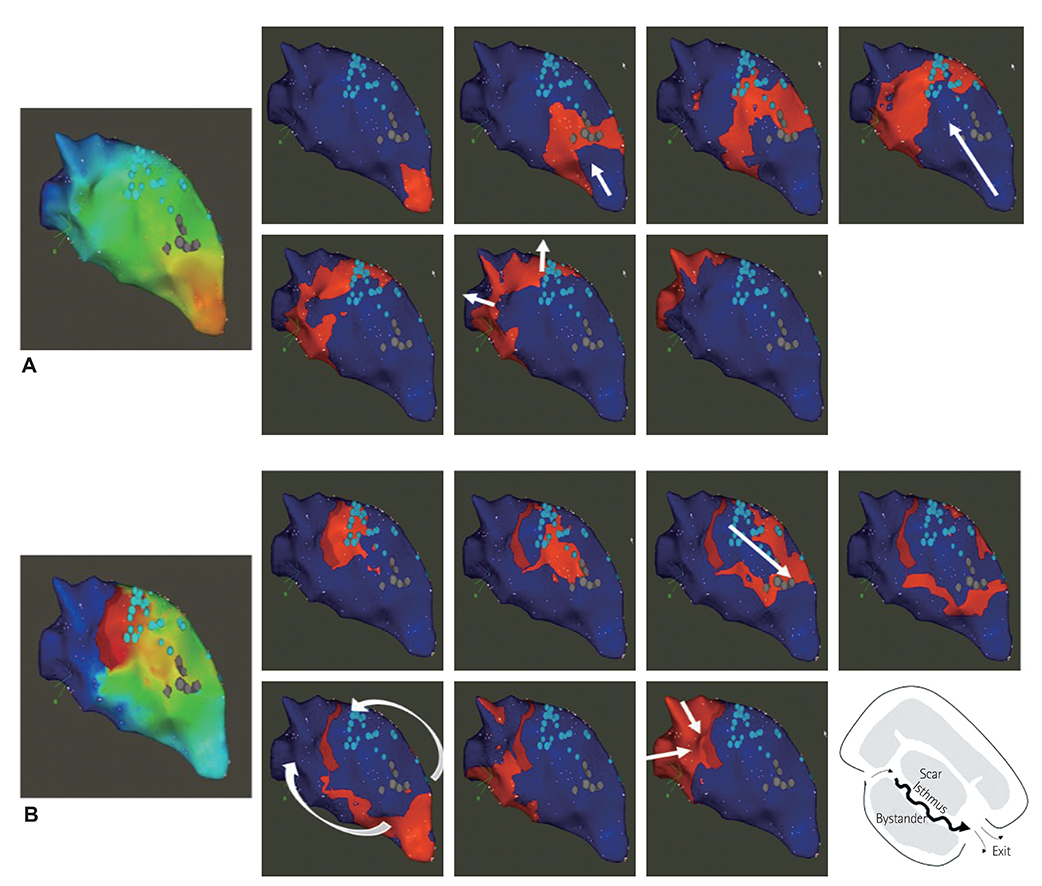
We analyzed five scar-related ventricular tachycardia cases. In the propagation maps, the resetting map showed the critical isthmus and entrance and exit sites of tachycardia that showed figure 8 reentry. However, conventional maps only showed the earliest ventricular activation sites and searched for focal tachycardia. All of the tachycardia cases were terminated by ablating the area around the isthmus.
CONCLUSION
Identifying the channel and direction of the critical isthmus by a new editing method to track delayed potential is essential in scar-related tachycardia.
MeSH Terms
Figure
Reference
-
1. Pedersen CT, Kay GN, Kalman J, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm. 2014; 11:e166–e196.2. Kuck KH. Should catheter ablation be the preferred therapy for reducing ICD shocks?: ventricular tachycardia in patients with an implantable defibrillator warrants catheter ablation. Circ Arrhythm Electrophysiol. 2009; 2:713–720.3. Morady F, Frank R, Kou WH, et al. Identification and catheter ablation of a zone of slow conduction in the reentrant circuit of ventricular tachycardia in humans. J Am Coll Cardiol. 1988; 11:775–782.4. Stevenson WG, Khan H, Sager P, et al. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993; 88(4 Pt 1):1647–1670.5. de Chillou C, Magnin-Poull I, Andronache M, et al. Showing up channels for postinfarct ventricular tachycardia ablation. Pacing Clin Electrophysiol. 2012; 35:897–904.6. Stevenson WG, Friedman PL, Sager PT, et al. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J Am Coll Cardiol. 1997; 29:1180–1189.7. de Chillou C, Lacroix D, Klug D, et al. Isthmus characteristics of reentrant ventricular tachycardia after myocardial infarction. Circulation. 2002; 105:726–731.8. Arenal A, Glez-Torrecilla E, Ortiz M, et al. Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease. J Am Coll Cardiol. 2003; 41:81–92.9. Jaïs P, Maury P, Khairy P, et al. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012; 125:2184–2196.10. Vergara P, Trevisi N, Ricco A, et al. Late potentials abolition as an additional technique for reduction of arrhythmia recurrence in scar related ventricular tachycardia ablation. J Cardiovasc Electrophysiol. 2012; 23:621–627.11. Mountantonakis SE, Park RE, Frankel DS, et al. Relationship between voltage map "channels" and the location of critical isthmus sites in patients with post-infarction cardiomyopathy and ventricular tachycardia. J Am Coll Cardiol. 2013; 61:2088–2095.12. Verma A, Marrouche NF, Schweikert RA, et al. Relationship between successful ablation sites and the scar border zone defined by substrate mapping for ventricular tachycardia post-myocardial infarction. J Cardiovasc Electrophysiol. 2005; 16:465–471.13. Haqqani HM, Marchlinski FE. Electrophysiologic substrate underlying postinfarction ventricular tachycardia: characterization and role in catheter ablation. Heart Rhythm. 2009; 6:8 Suppl. S70–S76.14. Vergara P, Roque C, Oloriz T, Mazzone P, Della Bella P. Substrate mapping strategies for successful ablation of ventricular tachycardia: a review. Arch Cardiol Mex. 2013; 83:104–111.15. de Chillou C, Groben L, Magnin-Poull I, et al. Localizing the critical isthmus of postinfarct ventricular tachycardia: the value of pace-mapping during sinus rhythm. Heart Rhythm. 2014; 11:175–181.16. Issa ZF, Miller JM, Zipes DP. Clinical Arrhythmology and Electrophysiology : A Companion to Braunwald's Heart Disease. 2nd ed. Philadelphia, PA: Elseiver/Saunders;2012. p. 537.17. Fahmy TS, Wazni OM, Jaber WA, et al. Integration of positron emission tomography/computed tomography with electroanatomical mapping: a novel approach for ablation of scar-related ventricular tachycardia. Heart Rhythm. 2008; 5:1538–1545.18. Di Biase L, Santangeli P, Burkhardt DJ, et al. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2012; 60:132–141.19. de Bakker JM, Wittkampf FH. The pathophysiologic basis of fractionated and complex electrograms and the impact of recording techniques on their detection and interpretation. Circ Arrhythm Electrophysiol. 2010; 3:204–213.20. Nakahara S, Tung R, Ramirez RJ, et al. Distribution of late potentials within infarct scars assessed by ultra high-density mapping. Heart Rhythm. 2010; 7:1817–1824.21. Yokokawa M, Desjardins B, Crawford T, Good E, Morady F, Bogun F. Reasons for recurrent ventricular tachycardia after catheter ablation of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2013; 61:66–73.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of broad QRS paroxysmall supraventricular tachycardia that is difficult to differentiate from ventricular tachycardia
- Huge Multilobulated Left Ventricular Outflow Tract Pseudoaneurysm Presenting with Ventricular Tachycardia
- Nonsustained ventricular tachycardia during outpatient anesthesia: a case report
- Clinical Study of Antiarrhythmic Effect of Mexiletine
- Pitfalls of Atrial Advancement Using a Ventricular Extra-stimulus During Supraventricular Tachycardia