J Rheum Dis.
2014 Dec;21(6):303-310. 10.4078/jrd.2014.21.6.303.
A Meta-analysis of the Diagnostic Value of Minor Salivary Gland Biopsy for Primary Sjogren's Syndrome
- Affiliations
-
- 1Division of Rheumatology, Korea University Medical Center, Division of Rheumatology, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. lyhcgh@korea.ac.kr
- KMID: 2222934
- DOI: http://doi.org/10.4078/jrd.2014.21.6.303
Abstract
OBJECTIVE
The purpose of this study is to evaluate the diagnostic performance of minor salivary gland biopsy (MSGB) for patients with primary Sjogren's syndrome (pSS).
METHODS
We have conducted a search from Medline, Embase, and Cochrane Library databases, and performed a meta-analysis on the diagnostic accuracy of MSGB in pSS patients.
RESULTS
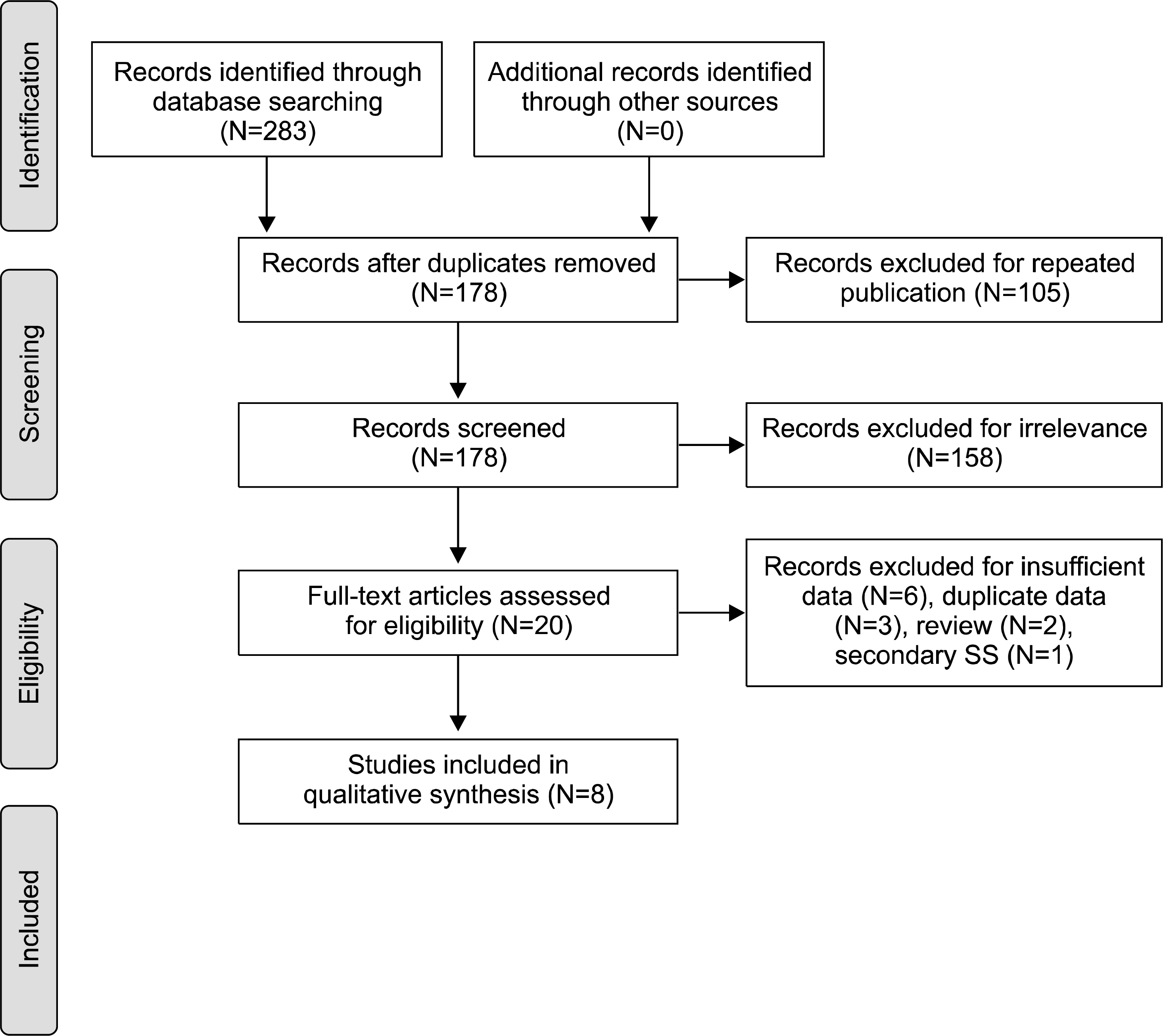
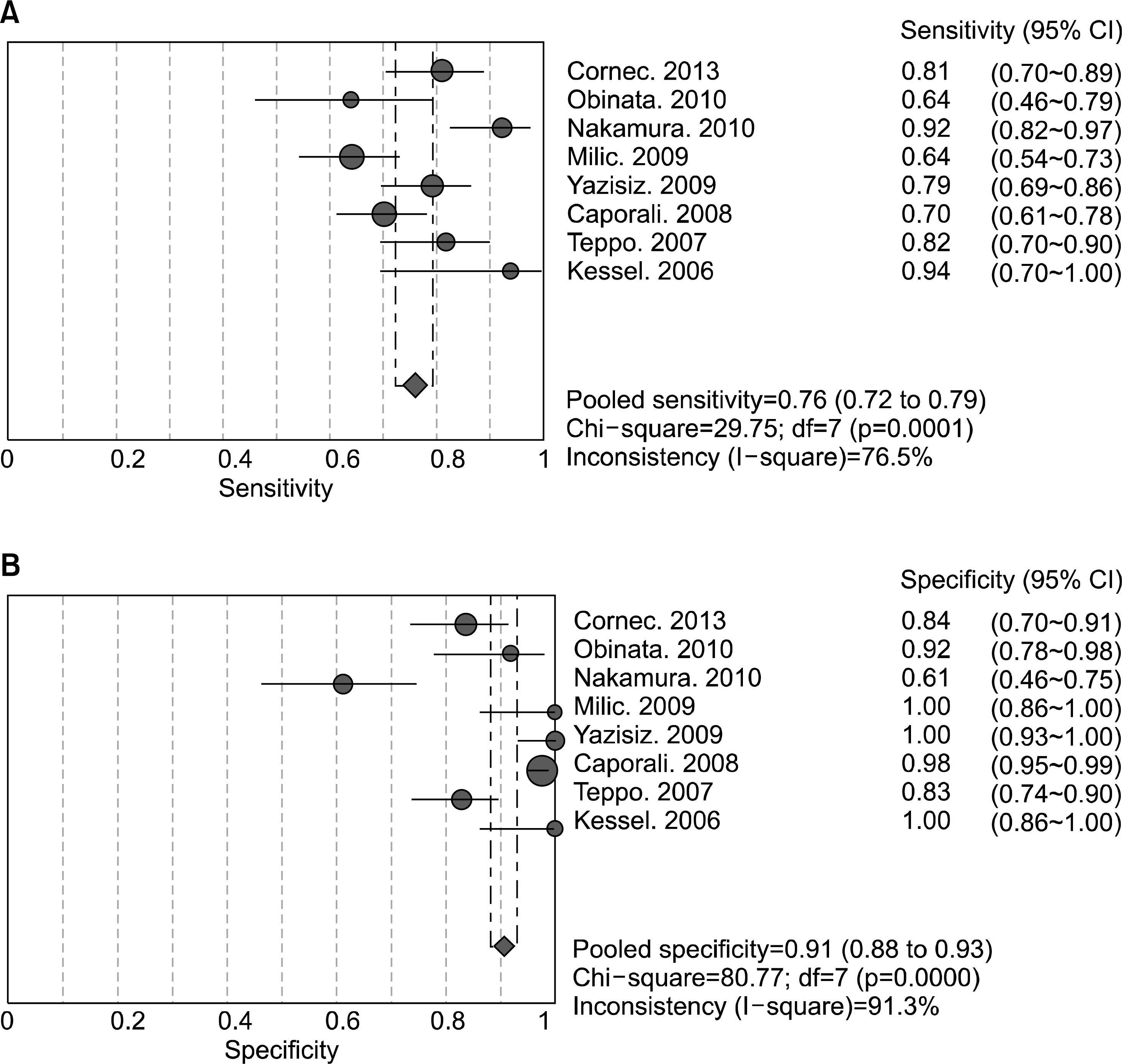
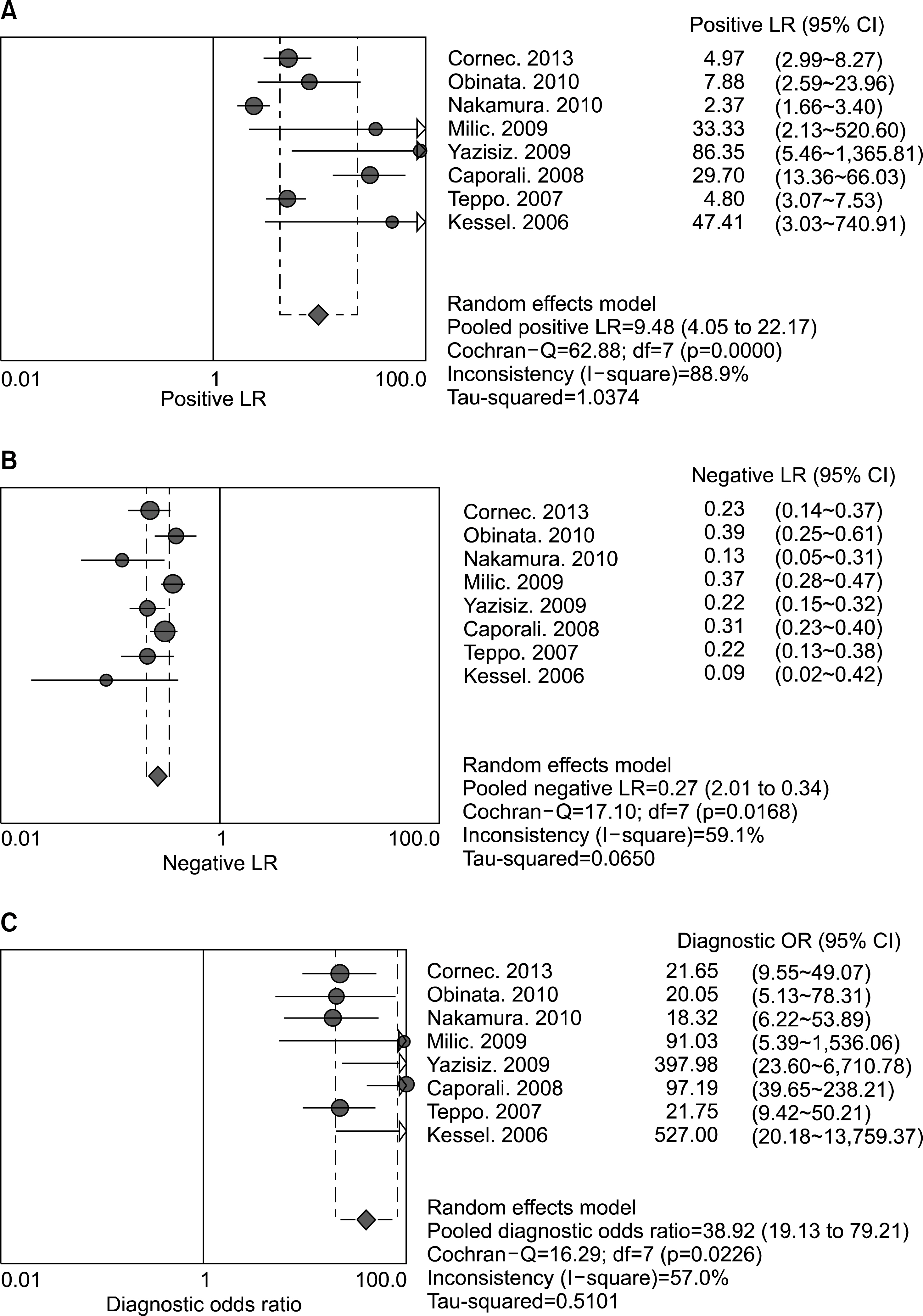
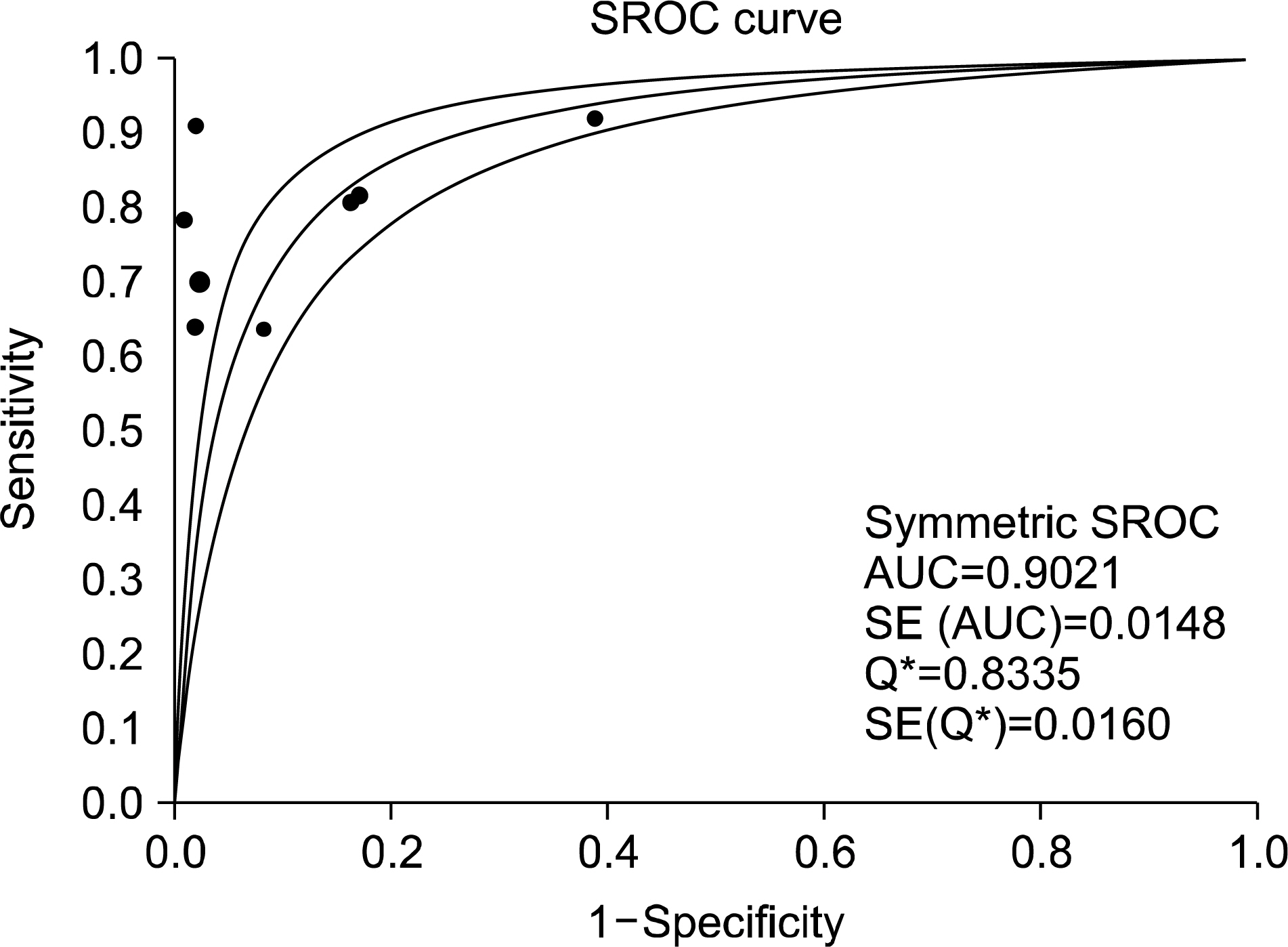
A total of eight studies, including 583 pSS and 627 non-pSS patients, were available for the meta-analysis. The pooled sensitivity and specificity of MSGB were 75.7% (95% confidence interval [CI], 72.0~79.1) and 90.7% (88.1~92.9), respectively. The positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio were 9.475 (4.051~22.16), 0.266 (0.208~0.340), and 38.92 (19.12~72.21), respectively. The area under the curve was 0.901 and the Q*; index was 0.902, indicating a high diagnostic accuracy. Some between-study heterogeneity was found in the meta-analyses; however, there was no evidence of a threshold effect (Spearman correlation coefficient= 0.419; p=0.301). Meta-regression showed that the study quality, sample size, study design, and diagnostic criteria were not sources of heterogeneity, and subgroup meta-analyses did not change the overall diagnostic accuracy.
CONCLUSION
Our meta-analysis of published studies demonstrates that MSGB has a high diagnostic accuracy and may play an important role in the diagnosis of pSS.
MeSH Terms
Figure
Reference
-
1. Kruszka P, O'Brian RJ. Diagnosis and management of Sjö gren syndrome. Am Fam Physician. 2009; 79:465–70.2. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. European Study Group on Classification Criteria for Sjö gren's Syndrome. Classification criteria for Sjö gren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002; 61:554–8.3. Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, et al. Sjö gren's International Collaborative Clinical Alliance (SICCA) Research Groups. American College of Rheumatology classification criteria for Sjö gren's syndrome: a da-ta-driven, expert consensus approach in the Sjö gren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken). 2012; 64:475–87.4. van Stein-Callenfels D, Tan J, Bloemena E, van Vugt RM, Voskuyl AE, Santana NT, et al. The role of a labial salivary gland biopsy in the diagnostic procedure for Sjö gren's syndrome; a study of 94 cases. Med Oral Patol Oral Cir Bucal. 2014; 19:e372–6.5. Cornec D, Jousse-Joulin S, Pers JO, Marhadour T, Cochener B, Boisramé-Gastrin S, et al. Contribution of salivary gland ultrasonography to the diagnosis of Sjö gren's syndrome: toward new diagnostic criteria? Arthritis Rheum. 2013; 65:216–25.6. Obinata K, Sato T, Ohmori K, Shindo M, Nakamura M. A comparison of diagnostic tools for Sjö gren syndrome, with emphasis on sialography, histopathology, and ultrasonography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:129–34.7. Nakamura H, Kawakami A, Iwamoto N, Okada A, Yamasaki S, Tamai M, et al. A single centre retrospective analysis of AECG classification criteria for primary Sjogren's syndrome based on 112 minor salivary gland biopsies in a Japanese population. Rheumatology (Oxford). 2010; 49:1290–3.
Article8. Milic VD, Petrovic RR, Boricic IV, Marinkovic-Eric J, Radunovic GL, Jeremic PD, et al. Diagnostic value of salivary gland ultrasonographic scoring system in primary Sjogren's syndrome: a comparison with scintigraphy and biopsy. J Rheumatol. 2009; 36:1495–500.9. Yazisiz V, Avci AB, Erbasan F, Kiriş E, Terzioğlu E. Diagnostic performance of minor salivary gland biopsy, serological and clinical data in Sjö gren's syndrome: a retrospective analysis. Rheumatol Int. 2009; 29:403–9.10. Caporali R, Bonacci E, Epis O, Bobbio-Pallavicini F, Morbini P, Montecucco C. Safety and usefulness of minor salivary gland biopsy: retrospective analysis of 502 procedures performed at a single center. Arthritis Rheum. 2008; 59:714–20.
Article11. Teppo H, Revonta M. A follow-up study of minimally invasive lip biopsy in the diagnosis of Sjö gren's syndrome. Clin Rheumatol. 2007; 26:1099–103.12. Kessel A, Toubi E, Rozenbaum M, Zisman D, Sabo E, Rosner I. Sjö gren's syndrome in the community: can serology replace salivary gland biopsy? Rheumatol Int. 2006; 26:337–9.13. Song GG, Lee YH. Diagnostic accuracies of sialography and salivary ultrasonography in Sjö gren's syndrome patients: a meta-analysis. Clin Exp Rheumatol. 2014; 32:516–22.14. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003; 3:25.
Article15. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–58.
Article16. Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997; 315:1533–7.
Article17. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–88.
Article18. Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med. 2002; 21:1525–37.
Article19. Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006; 6:31.
Article20. Goules AV, Tzioufas AG, Moutsopoulos HM. Classification criteria of Sjö gren's syndrome. J Autoimmun. 2014; 48-49:42–5.21. Egger M, Smith GD. Meta-Analysis. Potentials and promise. BMJ. 1997; 315:1371–4.
Article22. Guellec D, Cornec D, Jousse-Joulin S, Marhadour T, Marcorelles P, Pers JO, et al. Diagnostic value of labial minor salivary gland biopsy for Sjö gren's syndrome: a systematic review. Autoimmun Rev. 2013; 12:416–20.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Convenient and Less Invasive Technique of Labial Minor Salivary Gland Biopsy Using a Minimal Incision With a Needle Tip
- A Case of Sjogren Syndrome in Parotid Gland
- Two Cases of Acute Cerebral Infarction Associated with Primary Sjogren's Syndrome
- Mucosa-Associated Lymphoid Tissue Lymphoma of the Labial Minor Salivary Glands: Case Report
- Ultrasonography of the salivary glands in primary Sjogren's syndrome with positive anti Ro/SS-A antibody