Clin Exp Otorhinolaryngol.
2011 Sep;4(3):155-158.
Reversible Sensorineural Hearing Loss due to Pachymeningitis Associated with Elevated Serum MPO-ANCA
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Daegu Fatima Hospital, Daegu, Korea.
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Kyungpook National University School of Medicine, Daegu, Korea. leeshu@mail.knu.ac.kr
- 3Division of Rheumatology, Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea.
Abstract
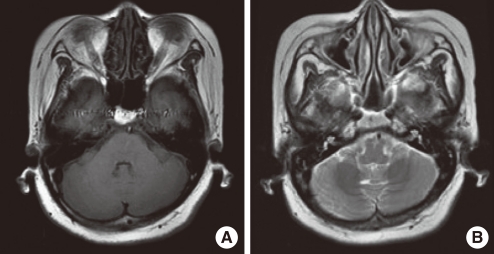
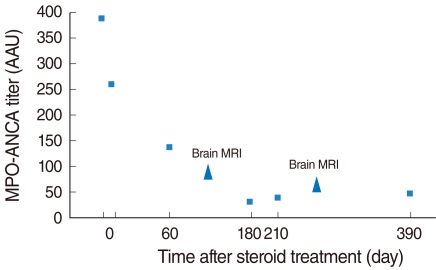
- Hypertrophic pachymeningitis is a progressive disease resulting in a diffuse thickening of dura mater due to inflammation, tumor or autoimmune diseases, but most cases are idiopathic. It is seldom reported to be related to sensorineural hearing loss, but it can cause sensorineural hearing loss which can be potentially reversed through treatment. Here, we report the case of a 54-year-old woman who had progressive, bilateral, worse in the left, sensorineural hearing loss and visual disturbance with an accompanying headache over several months. Brain MRI showed diffusely thickened dura mater, highly enhanced after gadolinium administration, which was consistent with pachymeningitis. It was assumed to be related to autoimmune pathogenesis on the basis of elevated serum myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA) titers. After empirical steroid and cyclophosphamide therapy, auditory impairment improved, especially in the high frequency region of the pure tone audiogram, and significant improvement in the word recognition test. Moreover, a follow-up MRI revealed much decreased enhancement of the dura mater, and the MPO-ANCA titer decreased to within the normal range. In the case of rapidly progressive sensorineural hearing loss or hearing impairment accompanying other cranial neuropathy, pachymeningitis should be taken into consideration, and brain MRI with gadolinium enhancement is the best method of detecting it. Also, to ensure proper treatment, a cautious evaluation including an ANCA work-up should be performed.
Keyword
MeSH Terms
-
Antibodies, Antineutrophil Cytoplasmic
Autoimmune Diseases
Brain
Cranial Nerve Diseases
Cyclophosphamide
Cytoplasm
Dietary Sucrose
Dura Mater
Female
Follow-Up Studies
Gadolinium
Headache
Hearing Loss
Hearing Loss, Sensorineural
Humans
Inflammation
Meningitis
Middle Aged
Reference Values
Wegener Granulomatosis
Antibodies, Antineutrophil Cytoplasmic
Cyclophosphamide
Dietary Sucrose
Gadolinium
Figure
Reference
-
1. Ruckenstein MJ. Autoimmune inner ear disease. Curr Opin Otolaryngol Head Neck Surg. 2004; 10. 12(5):426–430. PMID: 15377956.
Article2. Harris JP, Weisman MH, Derebery JM, Espeland MA, Gantz BJ, Gulya AJ, et al. Treatment of corticosteroid-responsive autoimmune inner ear disease with methotrexate: a randomized controlled trial. JAMA. 2003; 10. 08. 290(14):1875–1883. PMID: 14532316.3. Kupersmith MJ, Martin V, Heller G, Shah A, Mitnick HJ. Idiopathic hypertrophic pachymeningitis. Neurology. 2004; 3. 09. 62(5):686–694. PMID: 15007115.
Article4. Oghalai JS, Ramirez AL, Hegarty JL, Jackler RK. Chronic pachymeningitis presenting as asymmetric sensorineural hearing loss. Otol Neurotol. 2004; 7. 25(4):616–621. PMID: 15241244.
Article5. Iwasaki S, Matsui Y, Ito K, Naito R, Abbey K. Hypertrophic cranial pachymeningitis presenting as steroid-responsive hearing loss. Ann Otol Rhinol Laryngol. 2003; 5. 112(5):476–479. PMID: 12784990.
Article6. Bovo R, Berto A, Palma S, Ceruti S, Martini A. Symmetric sensorineural progressive hearing loss from chronic idiopathic pachymeningitis. Int J Audiol. 2007; 2. 46(2):107–110. PMID: 17365062.
Article7. Hatano N, Behari S, Nagatani T, Kimura M, Ooka K, Saito K, et al. Idiopathic hypertrophic cranial pachymeningitis: clinicoradiological spectrum and therapeutic options. Neurosurgery. 1999; 12. 45(6):1336–1342. PMID: 10598701.
Article8. Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997; 11. 20. 337(21):1512–1523. PMID: 9366584.
Article9. Nagashima T, Maguchi S, Terayama Y, Horimoto M, Nemoto M, Nunomura M, et al. P-ANCA-positive Wegener's granulomatosis presenting with hypertrophic pachymeningitis and multiple cranial neuropathies: case report and review of literature. Neuropathology. 2000; 3. 20(1):23–30. PMID: 10935433.
Article10. Fam AG, Lavine E, Lee L, Perez-Ordonez B, Goyal M. Cranial pachymeningitis: an unusual manifestation of Wegener's granulomatosis. J Rheumatol. 2003; 9. 30(9):2070–2074. PMID: 12966618.11. Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988; 6. 23. 318(25):1651–1657. PMID: 2453802.
Article12. Takagi D, Nakamaru Y, Maguchi S, Furuta Y, Fukuda S. Clinical features of bilateral progressive hearing loss associated with myeloperoxidase-antineutrophil cytoplasmic antibody. Ann Otol Rhinol Laryngol. 2004; 5. 113(5):388–393. PMID: 15174767.
Article13. Rojana-udomsart A, Pulkes T, Viranuwatti K, Laothamatas J, Phudhichareonrat S, Witoonpanich R. Idiopathic hypertrophic cranial pachymeningitis. J Clin Neurosci. 2008; 4. 15(4):465–469. PMID: 18249120.
Article14. Lam BL, Barrett DA, Glaser JS, Schatz NJ, Brown HH. Visual loss from idiopathic intracranial pachymeningitis. Neurology. 1994; 4. 44(4):694–698. PMID: 8164828.
Article15. Jerger J, Hayes D. Diagnostic speech audiometry. Arch Otolaryngol. 1977; 4. 103(4):216–222. PMID: 849199.
Article16. Rizzo SR Jr, Gutnick HN. Cochlear versus retrocochlear presbyacusis: clinical correlates. Ear Hear. 1991; 2. 12(1):61–63. PMID: 2026290.17. Korsan-Bengtsen M. Distorted speech audiometry: a methodological and clinical study. Acta Otolaryngol Suppl. 1973; 310:1–75. PMID: 4521066.18. Mcardle R, Hnath-Chisolm T. Katz J, Medwetsky L, Burkard R, Hood LJ, editors. Speech audiometry. Handbook of clinical audiology. 2009. 6th ed. Baltimore (MD): Williams & Wilkins;p. 64–79.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rehabilitation of Sensorineural Hearing Loss: Hearing Aid
- A Case of Wegener's Granulomatosis with Periaortitis and Pachymeningitis
- The Characteristics and the Changes of Tinnitus according to the Recovery of Hearing Loss in the Patients with Sudden Hearing Loss
- Cochleovestibular Otosclerosis Without Conductive Hearing Loss
- A Case of Sensorineural Hearing Loss Caused by Neurosyphilis in Patient Who Is Treated by Anti-Interleukin 17A Monoclonal Antibody