J Korean Ophthalmol Soc.
2013 Nov;54(11):1788-1793. 10.3341/jkos.2013.54.11.1788.
Thrombocytopenia after Intravitreal Bevacizumab Injection for Macular Edema in Branch Retinal Vein Occlusion
- Affiliations
-
- 1Department of Ophthalmology, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Ilsan, Korea. ophtha@naver.com
- KMID: 2217929
- DOI: http://doi.org/10.3341/jkos.2013.54.11.1788
Abstract
- PURPOSE
To report a rare case of thrombocytopenia after intravitreal bevacizumab injection (IVBI) in a patient with macular edema secondary to branch retinal vein occlusion (BRVO).
CASE SUMMARY
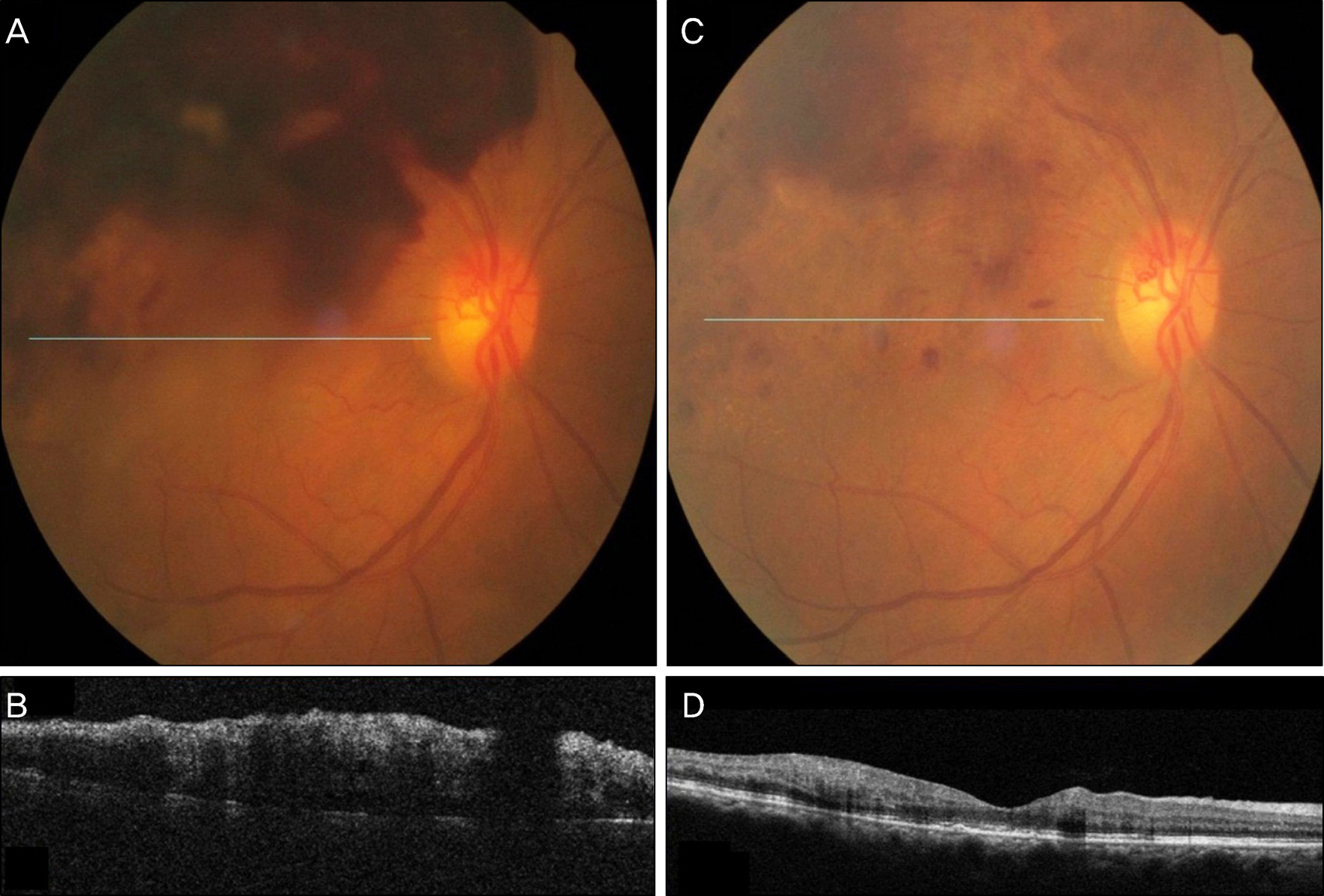
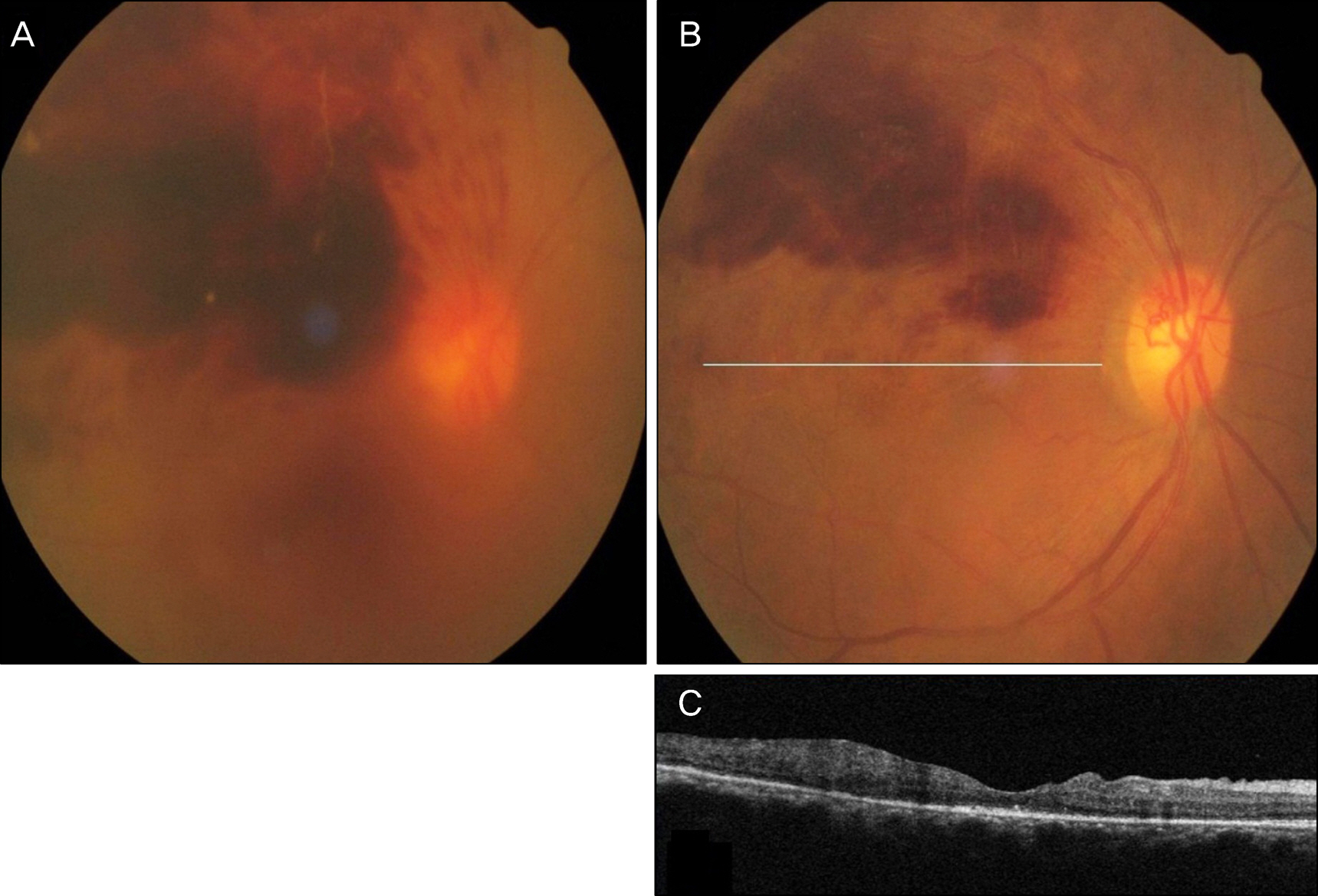
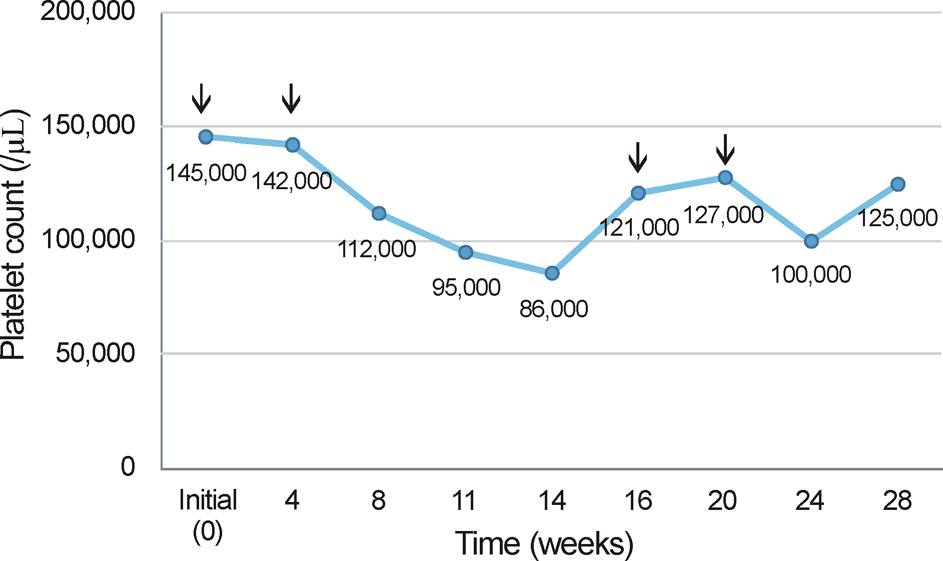
A 52-year-old female presented to our clinic with complaints of visual disturbance in her right eye for 2 months. She was receiving hemodialysis therapy 3 times a week for 4 years due to chronic renal failure. Unilateral macular edema secondary to BRVO was observed on fundus examination and was confirmed with optical coherence tomography (OCT). The first IVBI was performed, and an additional injection was given 4 weeks later. Four weeks after the second injection, thrombocytopenia was present. The patient was followed up in our clinic without IVBI for 8 weeks and the platelet count recovered. Thrombocytopenia was reconfirmed after 2 additional monthly injections. After she revisited our clinic without IVBI for 8 weeks, the platelet count recovered without any treatment.
CONCLUSIONS
When a patient presents with thrombocytopenia after IVBI with macular edema in BRVO, thrombocytopenia due to IVBI should be considered as a possible diagnosis.
MeSH Terms
Figure
Reference
-
References
1. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and devel- opment of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004; 3:391–400.2. Schutz FA, Jardim DL, Je Y, Choueiri TK. Haematologic toxicities associated with the addition of bevacizumab in cancer patients. Eur J Cancer. 2011; 47:1161–74.
Article3. Kazazi-Hyseni F, Beijnen JH, Schellens JH. Bevacizumab. Oncologist. 2010; 15:819–25.
Article4. Ranpura V, Hapani S, Wu S. Treatment-related mortality with bev- acizumab in cancer patients: a meta-analysis. JAMA. 2011; 305:487–94.5. Astam N, Batioglu F, Ozmert E. Short-term efficacy of intravitreal bevacizumab for the treatment of macular edema due to diabetic retinopathy and retinal vein occlusion. Int Ophthalmol. 2009; 29:543–8.
Article6. Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bev- acizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006; 113:1695.e1–15.7. Funk M, Kriechbaum K, Prager F, et al. Intraocular concentrations of growth factors and cytokines in retinal vein occlusion and the ef- fect of therapy with bevacizumab. Invest Ophthalmol Vis Sci. 2009; 50:1025–32.8. Kreutzer TC, Alge CS, Wolf AH, et al. Intravitreal bevacizumab for the treatment of macular oedema secondary to branch retinal vein occlusion. Br J Ophthalmol. 2008; 92:351–5.
Article9. Mandal S, Venkatesh P, Sampangi R, Garg S. Intravitreal bev- acizumab (Avastin) as primary treatment for myopic choroidal neovascularization. Eur J Ophthalmol. 2007; 17:620–6.10. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350:2335–42.
Article11. Leal T, Robins HI. Bevacizumab induced reversible thrombocyto- penia in a patient with recurrent high-grade glioma: a case report. Cancer Chemother Pharmacol. 2010; 65:399–401.12. Kumar J, Bhargava M, Aggarwal S. Bevacizumab-induced rever- sible thrombocytopenia in a patient with adenocarcinoma of colon: rare adverse effect of bevacizumab. Case Rep Oncol Med. 2012; 2012:695430.13. Dior M, Coriat R, Mir O, et al. A rare hematological adverse event induced by bevacizumab: severe thrombocytopenia. Am J Med. 2012; 125:828–30.
Article14. Day S, Acquah K, Mruthyunjaya P, et al. Ocular complications af- ter anti-vascular endothelial growth factor therapy in Medicare pa- tients with age-related macular degeneration. Am J Ophthalmol. 2011; 152:266–72.15. Gordon MS, Cunnigham D. Managing patients treated with bava- cizumab combination therapy. Oncology. 2005; 69(Suppl 3):25–33.16. Skillings JR, Johnson DH, Miller K, et al. Arterial thromboembolic events (ATEs) is a pooled analysis of 5 randomized, controlled tri- als (RCTs) of bevacizumab (BV) with chemotherapy. J Clin Oncol. 2005; 23:3019.17. Fung AE, Rosenfeld PJ, Reichel E. The international intravitreal bevacizumab safety survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006; 90:1344–9.
Article18. Comstock TJ. Renal dialysis. Young LY, Koda-Kimble MA, editors. Applied Therapeutics: The Clinical Use of Drugs. 6th ed.Vancouver: WA;1995. 31:chap. 1-15.19. Warkentin TE, Kelton JG. A 14-year study of heparin-induced thrombocytopenia. Am J Med. 1996; 101:502–7.
Article20. Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced throm- bocytopenia in patients treated with low molecular weight heparin or unfractionated heparin. N Engl J. 1995; 332:1330–5.21. Hu Y, Yuan M, Lu X. Thrombocytopenia induced by both aspirin and clopidogrel in the same patient. Int J Clin Pharmacol Ther. 2013; 51:228–31.
Article22. Matsuyama K, Ogata N, Matsuoka M, et al. Plasma levels of vascular endothelial growth factor and pigment epithelium-derived factor before and after intravitreal injection of bevacizumab. Br J Ophthalmol. 2010; 94:1215–8.
Article23. Kut C, Mac Gabhann F, Popel AS. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer. 2007; 97:978–85.
Article24. Verheul HM, Lolkema MP, Qian DZ, et al. Platelets take up the monoclonal antibody bevacizumab. Clin Can Res. 2007; 13:5341–7.
Article25. Meyer T, Robles-Carrilo L, Robson T, et al. Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J Thromb Haemost. 2009; 7:171–81.
Article26. Curtis BR, Kaliszewski J, Marques MB, et al. Immune-mediated thrombocytopenia resulting from sensitivity to oxaliplatin. Am J Hematol. 2006; 81:193–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Macular Vessel Density Analysis Using Optical Coherence Tomography Angiography before and after Intravitreal Bevacizumab Injection in Branch Retinal Vein Occlusion
- The Efficacy of Intravitreal Bevacizumab in the Treatment of Macular Edema
- Effects of Intravitreal Bevacizumab Injection in 3 Types of Macular Edema Secondary to Branch Retinal Vein Occlusion
- Short-term Effects of Intravitreal Bevacizumab Injection and Macular Edema Patterns in Branch Retinal Vein Occlusion
- Intravitreal Bevacizumab Injection for Macular Edema Secondary to Branch Retinal Vein Occlusion: Long-Term Results