J Korean Ophthalmol Soc.
2012 Sep;53(9):1236-1246. 10.3341/jkos.2012.53.9.1236.
Comparison of Permanent Amniotic Membrane Transplantation and Temporary Amniotic Membrane Patch after Primary Pterygium Excision
- Affiliations
-
- 1Department of Ophthalmology, Yeungnam University College of Medicine, Daegu, Korea. sbummlee@ynu.ac.kr
- KMID: 2216075
- DOI: http://doi.org/10.3341/jkos.2012.53.9.1236
Abstract
- PURPOSE
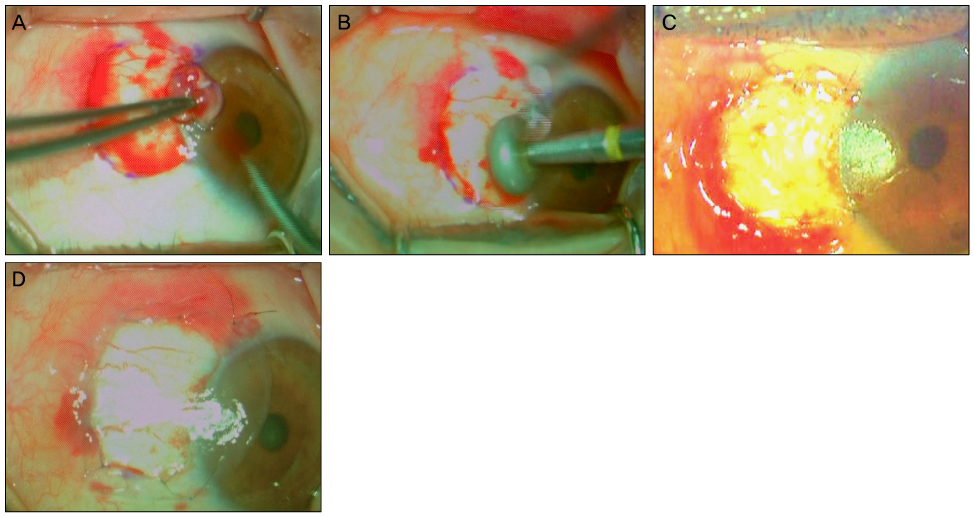
To compare the clinical results, recurrence rates, and recurrence-related risk factors of permanent amniotic membrane transplantation (PAMT, group 1) and temporary amniotic membrane patch (TAMP, group 2) after excision of primary pterygium.
METHODS
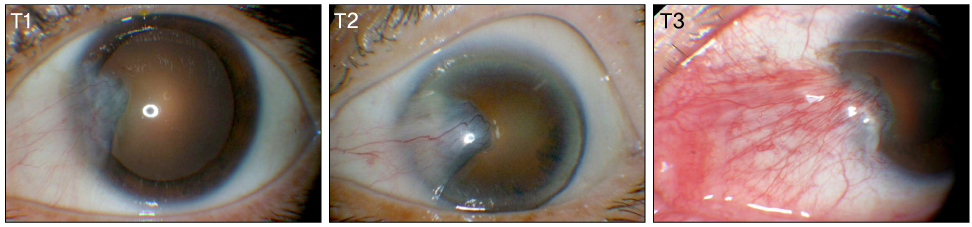
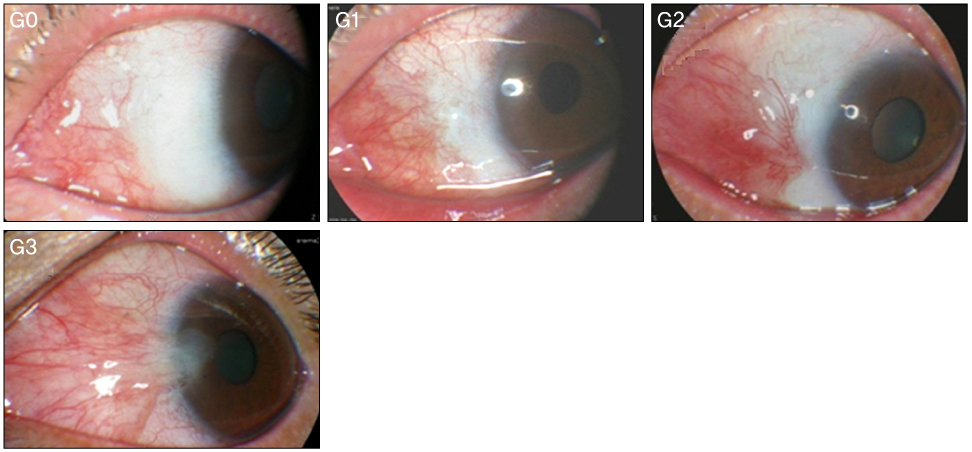
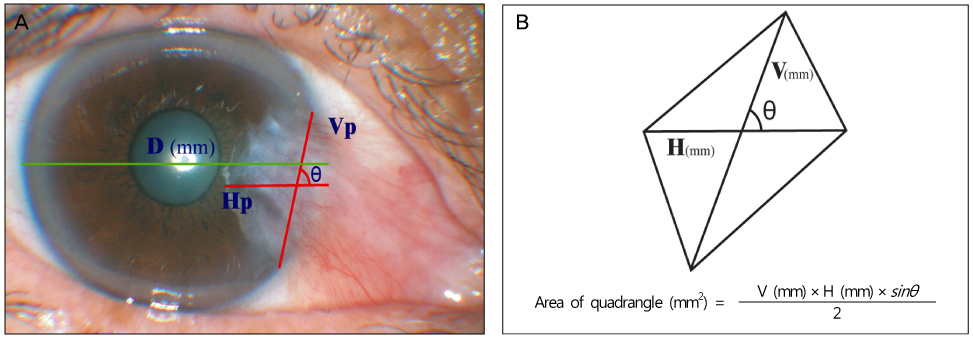
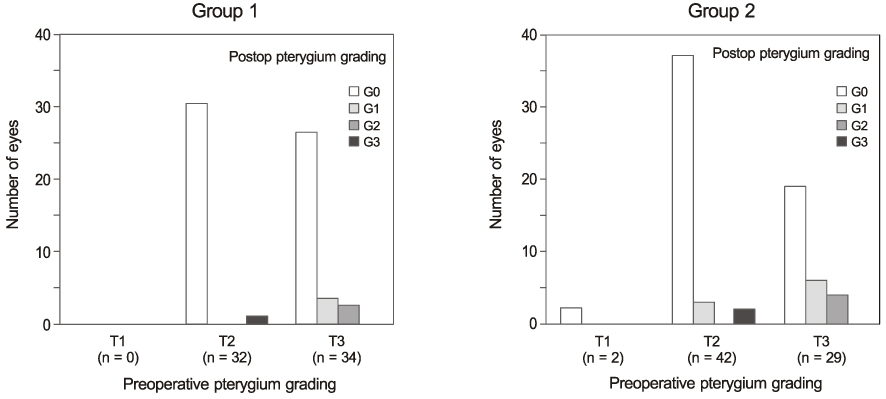
Recurrence grades were evaluated for 66 eyes (T1, T2, and T3; 0, 32, and 34 eyes, respectively) in group 1 and 73 eyes (T1, T2, and T3; 2, 42, and 29 eyes, respectively) in group 2. Surgical results were classified into surgical success (G0 or G1), conjunctival recurrence (G2), and corneal recurrence (G3). Recurrence rates were analyzed based on gender, age, Tan's preoperative grading system, horizontal and vertical length of the preoperative pterygium, the corneal involvement size of the preoperative pterygium, and epithelial healing time.
RESULTS
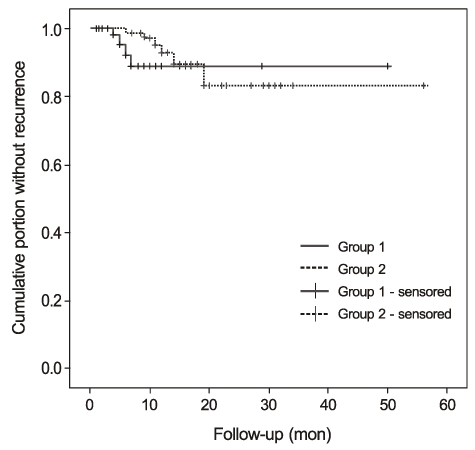
In the postoperative grading, 3 eyes (4.5%) and 1 eye (1.5%) in group 1, and 4 eyes (5.5%) and 2 eyes (2.7%) in group 2 were graded as clinically recurrence-occurred G2 and G3, respectively. There was no statistically significant difference in recurrence-occurred G2 and G3 cases between the two groups (p = 0.62). No risk factors of clinically significant G2 and G3 recurrence were identified in either group by Cox proportional hazards survival regression analysis.
CONCLUSIONS
The results of the present study suggest that PAMT tends to lower the recurrence rate compared to TAMP because the PAMT group had more T3 eyes than the TAMP group, although the two groups showed no statistically significant difference in clinically significant recurrence after pterygium excision.
Keyword
Figure
Cited by 1 articles
-
Short-Term Result of Triple Procedure in Pterygium Surgery
Yong Joon Kim, Jin Kwon Chung
J Korean Ophthalmol Soc. 2014;55(3):354-360. doi: 10.3341/jkos.2014.55.3.354.
Reference
-
1. Solomon A, Pires RT, Tseng SC. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology. 2001. 108:449–460.2. Verma N, Garap JA, Maris R, Kerek A. Intraoperative use of mitomycin C in the treatment of recurrent pterygium. P N G Med J. 1998. 41:37–42.3. Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997. 115:1235–1240.4. Riordan-Eva P, Kielhorn I, Ficker LA, et al. Conjunctival autografting in the surgical management of pterygium. Eye (Lond). 1993. 7(Pt 5):634–638.5. Panda A, Das GK, Tuli SW, Kumar A. Randomized trial of intraoperative mitomycin C in surgery for pterygium. Am J Ophthalmol. 1998. 125:59–63.6. Chen PP, Ariyasu RG, Kaza V, et al. A randomized trial comparing mitomycin C and conjunctival autograft after excision of primary pterygium. Am J Ophthalmol. 1995. 120:151–160.7. Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997. 104:974–985.8. Cho JW, Chung SH, Seo KY, Kim EK. Conjunctival mini-flap technique and conjunctival autotransplantation in pterygium surgery. J Korean Ophthalmol Soc. 2005. 46:1471–1477.9. Allan BD, Short P, Crawford GJ, et al. Pterygium excision with conjunctival autografting: an effective and safe technique. Br J Ophthalmol. 1993. 77:698–701.10. Luanratanakorn P, Ratanapakorn T, Suwan-Apichon O, Chuck RS. Randomised controlled study of conjunctival autograft versus amniotic membrane graft in pterygium excision. Br J Ophthalmol. 2006. 90:1476–1480.11. Küçükerdönmez C, Akova YA, Altinörs DD. Comparison of conjunctival autograft with amniotic membrane transplantation for pterygium surgery: surgical and cosmetic outcome. Cornea. 2007. 26:407–413.12. Solomon AS. Pterygium. Br J Ophthalmol. 2006. 90:665–666.13. Taylor HR. Aetiology of climatic droplet keratopathy and pterygium. Br J Ophthalmol. 1980. 64:154–163.14. Taylor HR, West S, Muñoz B, et al. The long-term effects of visible light on the eye. Arch Ophthalmol. 1992. 110:99–104.15. Coroneo MT. Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol. 1993. 77:734–739.16. Threlfall TJ, English DR. Sun exposure and pterygium of the eye: a dose-response curve. Am J Ophthalmol. 1999. 128:280–287.17. Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001. 119:695–706.18. Bradley JC, Yang W, Bradley RH, et al. The science of pterygia. Br J Ophthalmol. 2010. 94:815–820.19. Dushku N, Reid TW. P53 expression in altered limbal basal cells of pingueculae, pterygia, and limbal tumors. Curr Eye Res. 1997. 16:1179–1192.20. Kwok LS, Coroneo MT. A model for pterygium formation. Cornea. 1994. 13:219–224.21. Yang SF, Lin CY, Yang PY, et al. Increased expression of gelatinase (MMP-2 and MMP-9) in pterygia and pterygium fibroblasts with disease progression and activation of protein kinase C. Invest Ophthalmol Vis Sci. 2009. 50:4588–4596.22. Kria L, Ohira A, Amemiya T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygium. Acta Histochem. 1996. 98:195–201.23. Kria L, Ohira A, Amemiya T. Growth factors in cultured pterygium fibroblasts: immunohistochemical and ELISA analysis. Graefes Arch Clin Exp Ophthalmol. 1998. 236:702–708.24. Lee DH, Cho HJ, Kim JT, et al. Expression of vascular endothelial growth factor and inducible nitric oxide synthase in pterygia. Cornea. 2001. 20:738–742.25. Barraquer JI, Binder PS, Buxton JN. Etiology and treatment of pterygium. 1980. In : Symposium on Medical and Surgical Diseases of the Cornea. Transactions of the New Orleans Academy of Ophthalmology; St. Louis: Mosby;167–178.26. Kim CH, Lee JK, Park DJ. Recurrence rates of amniotic membrane transplantation, conjunctival autograft and conjunctivolimbal autograft in primary pterygium. J Korean Ophthalmol Soc. 2009. 50:1780–1788.27. Ozer A, Yildirim N, Erol N, Yurdakul S. Long-term results of bare sclera, limbal-conjunctival autograft and amniotic membrane graft techniques in primary pterygium excisions. Ophthalmologica. 2009. 223:269–273.28. Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989. 96:709–722.29. Al Fayez MF. Limbal versus conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 2002. 109:1752–1755.30. Kwak DY, Bae MC, Lee JK, Park DJ. Pterygium surgery: Wide excision with conjunctivo-limbal autograft. J Korean Ophthalmol Soc. 2008. 49:205–212.31. Ti SE, Tseng SC. Management of primary and recurrent pterygium using amniotic membrane transplantation. Curr Opin Ophthalmol. 2002. 13:204–212.32. Ma DH, See LC, Hwang YS, Wang SF. Comparison of amniotic membrane graft alone or combined with intraoperative mitomycin C to prevent recurrence after excision of recurrent pterygia. Cornea. 2005. 24:141–150.33. Mutlu FM, Sobaci G, Tatar T, Yildirim E. A comparative study of recurrent pterygium surgery: limbal conjunctival autograft transplantation versus mitomycin C with conjunctival flap. Ophthalmology. 1999. 106:817–821.34. Ti SE, Chee SP, Dear KB, Tan DT. Analysis of variation in success rates in conjunctival autografting for primary and recurrent pterygium. Br J Ophthalmol. 2000. 84:385–389.35. Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci U S A. 1996. 93:3509–3513.36. Detorakis ET, Zafiropoulos A, Arvanitis DA, Spandidos DA. Detection of point mutations at codon 12 of KI-ras in ophthalmic pterygia. Eye (Lond). 2005. 19:210–214.37. Detorakis ET, Zaravinos A, Spandidos DA. Growth factor expression in ophthalmic pterygia and normal conjunctiva. Int J Mol Med. 2010. 25:513–516.38. Ye J, Kook KH, Yao K. Temporary amniotic membrane patch for the treatment of primary pterygium: mechanisms of reducing the recurrence rate. Graefes Arch Clin Exp Ophthalmol. 2006. 244:583–588.39. Fernandes M, Sangwan VS, Bansal AK, et al. Outcome of pterygium surgery: analysis over 14 years. Eye (Lond). 2005. 19:1182–1190.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Amniotic Membrane Transplantation for Pterygium Excision
- Ocular Surface Reconstruction with Amniotic Membrane Transplantation in Pterygium
- Effects of Temporary Amniotic Membrane Patch after Surgical Excision of Primary Pterygium
- Recurrence Rates of Conjunctival Autograft Transplantation With Aminiotic Membrane Transplantation in Primary Pterygium Surgery
- Amniotic Membrane Transplantation for the Conjunctival Necrosis after Scleral Gra t in the Enucleated Eye: 1 Case Report