J Korean Ophthalmol Soc.
2016 Apr;57(4):533-539. 10.3341/jkos.2016.57.4.533.
Orbital Wall Reconstruction with Osteoconductive Unsintered Hydroxyapatite/Poly L-Lactide
- Affiliations
-
- 1Department of Ophthalmology, Gachon University Gil Medical Center, Incheon, Korea. cmj@gilhospital.com
- KMID: 2212784
- DOI: http://doi.org/10.3341/jkos.2016.57.4.533
Abstract
- PURPOSE
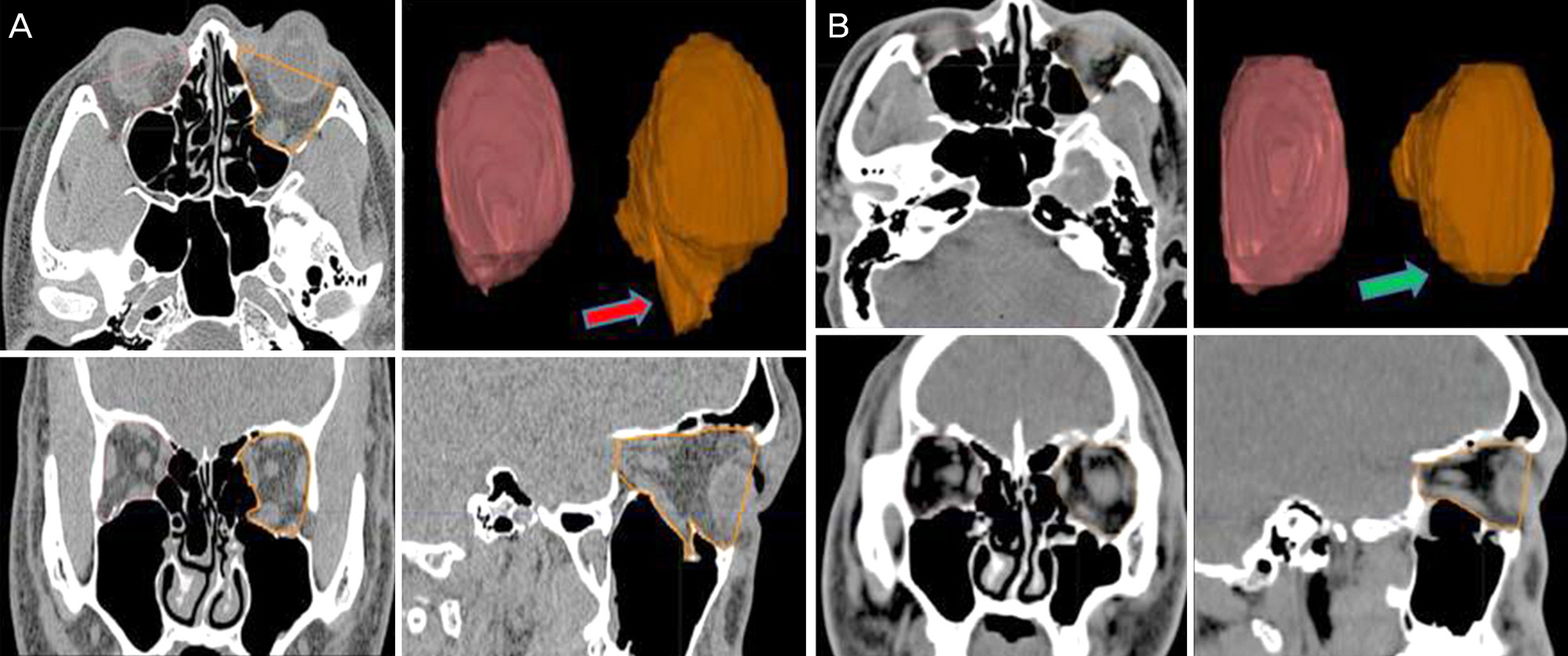
To evaluate the effect of orbital wall reconstruction with absorbable osteoconductive unsintered hydroxyapatite/poly L-lactide by assessment of the orbital volume via orbital computed tomography.
METHODS
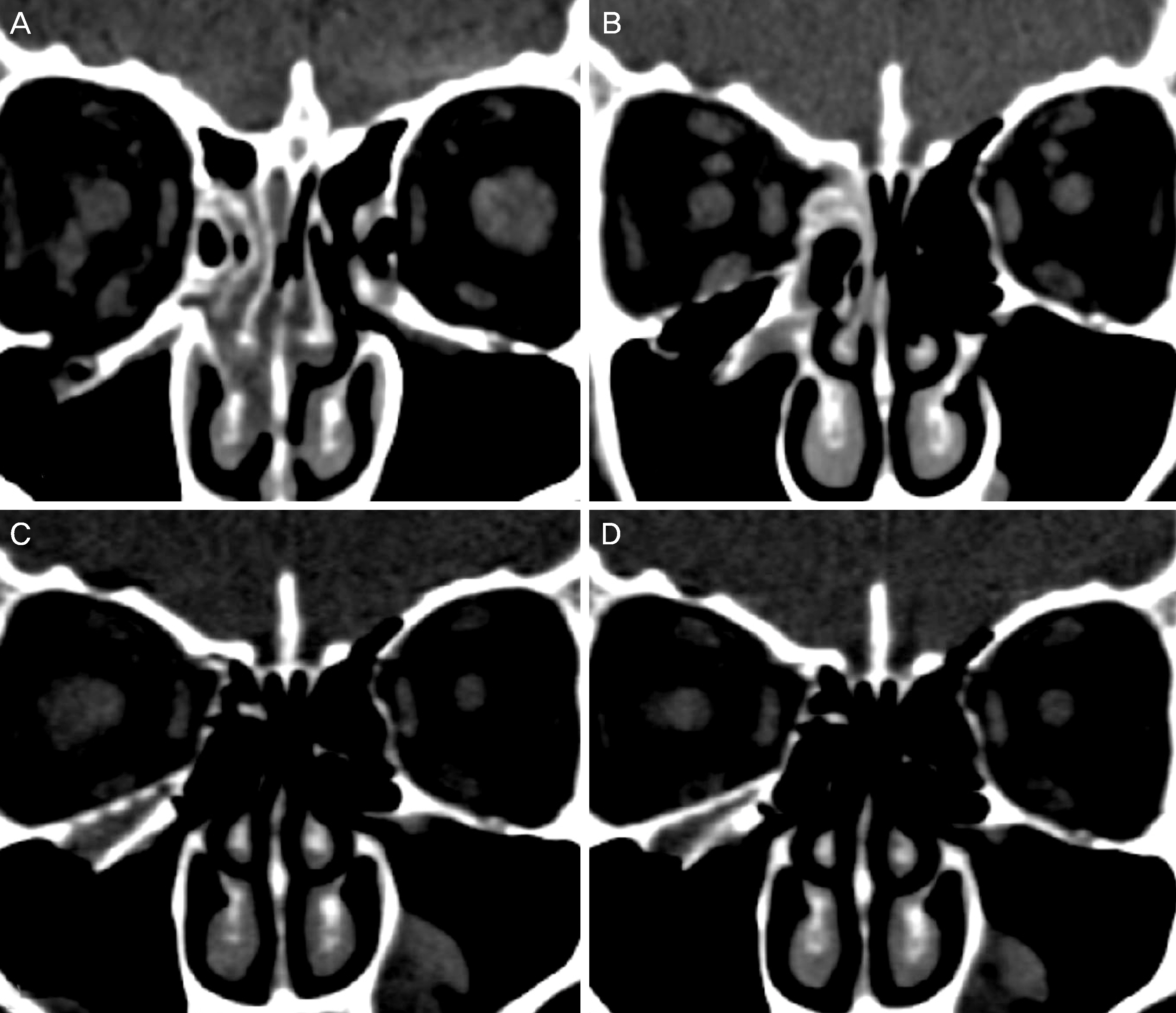
24 patients who followed up at least 6 months after orbital wall reconstruction with unsintered hydroxyapatite/poly L-lactide were included. Retrospective clinical chart reviews for clinical manifestations and complications were performed, and orbital volume measurements were taken using the Eclipse Treatment Planning System (ver.13.0, Varian Medical System Inc., Palo Alto, CA, USA) through orbital computed tomography, which were taken before operation, right after operation, and at last follow up.
RESULTS
Fourteen patients (58.3%) showed diplopia and extraocular muscle movement limitation preoperatively. Diplopia was resolved at last follow up and extraocular muscle movement limitation was improved at postoperative 6 months for all cases. The mean volumes of the fractured orbit and the unaffected orbit before operation were 23.62 ± 0.45 cm3 and 21.95 ± 1.01 cm3, respectively (p = 0.003). The mean volumes of the fractured orbit and the unaffected orbit right after operation were 21.65 ± 0.91 cm3 and 21.78 ± 0.83 cm3, respectively (p = 0.542). The mean volumes of the fractured orbit and the unaffected orbit at last follow up were 21.84 ± 0.93 cm3 and 21.81 ± 0.91 cm3, respectively (p = 0.889).
CONCLUSIONS
Absorbable osteoconductive unsintered hydroxyapatite/poly L-lactide was effective for clinical improvement and orbital volume assessment in cases of orbital wall reconstruction and it can be used safely without definite implant related complications.
Keyword
Figure
Reference
-
References
1. Jeon SY, Kim C, Ma Y, Hwang E. Microsurgical intranasal reconstruction of isolated blowout fractures of the medial orbital wall. Laryngoscope. 1996; 106:910–3.
Article2. Choi JC, Fleming JC, Aitken PA, Shore JW. Porous polyethylene channel implants: a modified porous polyethylene sheet implant designed for repairs of large and complex orbital wall fractures. Ophthal Plast Reconstr Surg. 1999; 15:56–66.3. Block MS, Kent JN. Correction of vertical orbital dystopia with a hydroxyapatite orbital floor graft. J Oral Maxillofac Surg. 1988; 46:420–5.4. Jordan DR, St Onge P, Anderson RL, et al. Complications associated with alloplastic implants used in orbital fracture repair. Ophthalmology. 1992; 99:1600–8.
Article5. Scales JT. Discussion on metals and synthetic materials in relation to soft tissues; Tissue reaction to synthetic materials. Proc R Soc Med. 1953; 46:647.6. Shikinami Y, Matsusue Y, Nakamura T. The complete process of bioresorption and bone replacement using devices made of forged composites of raw hydroxyapatite particles/poly l-lactide (F-u-HA/PLLA). Biomaterials. 2005; 26:5542–51.7. Shikinami Y, Okuno M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly-L-lactide (PLLA): Part I. Basic characteristics. Biomaterials. 1999; 20:859–77.8. Shikinami Y, Okuno M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly L-lactide (PLLA). Part II: practical properties of miniscrews and miniplates. Biomaterials. 2001; 22:3197–211.
Article9. Oum BS. Eye Exam. 3rd ed.Goyang: Naewaehaksool;2013. p. 146–7.10. Mesbahi A, Thwaites DI, Reilly AJ. Experimental and Monte Carlo evaluation of eclipse treatment planning system for lung dose calculations. Rep Pract Oncol Radiother. 2006; 11:123–33.
Article11. Ye J, Kook KH, Lee SY. Evaluation of computer-based volume measurement and porous polyethylene channel implants in reconstruction of large orbital wall fractures. Invest Ophthalmol Vis Sci. 2006; 47:509–13.
Article12. Matsuda N, Kaji F. Form control of crystals and aggregation of hydroxyapatites. Kokubo T, Nakamura T, Miyaji F, editors. Bioceramics. Oxford: Pergamon Press;1996. 9:chap. 9.13. Ducheyne P, Qiu Q. Bioactive ceramic: the effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999; 20:2287–303.14. Matsusue Y, Niibayashi H, Aoki Y. Bone fusion using HA/PLLA composite, a material characterized by its excellent resorbability in the living body. J Orthop Surg (Hong Kong). 1999; 50:1405–11.15. Hayashi M, Muramatsu H, Sato M, et al. Surgical treatment of facial fracture by using unsintered hydroxyapatite particles/poly l-lactide composite device (OSTEOTRANS MX(R)): a clinical study on 17 cases. J Craniomaxillofac Surg. 2013; 41:783–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medial Wall Orbital Reconstruction using Unsintered Hydroxyapatite Particles/Poly L-Lactide Composite Implants
- Unsintered Hydroxyapatite and Poly-L-Lactide Composite Screws/Plates for Stabilizing β-Tricalcium Phosphate Bone Implants
- Biodegradable implants for orbital wall fracturereconstruction
- The Surgical Results of Isolated Orbital Blowout Fractures Using Bioresorbable Poly L-/DL-Lactide 70/30 Implant
- Clinical analysis in reconstruction of orbital blow-out fracture using the hydroxyapatite