J Korean Ophthalmol Soc.
2008 Mar;49(3):401-408. 10.3341/jkos.2008.49.3.401.
The Short-Term Effect of Topical Cyclosporine A 0.05% in Various Ocular Surface Disorder
- Affiliations
-
- 1The Institute of Vision Research, Department of Ophthalmology, College of Medicine, Yonsei University, Seoul, Korea. seoky@yumc.yonsei.ac.kr
- KMID: 2211312
- DOI: http://doi.org/10.3341/jkos.2008.49.3.401
Abstract
-
PURPOSE: To evaluate the efficacy of topical cyclosporine A 0.05% (Restasis) in the treatment of dry eye symptoms caused by various ocular surface inflammatory disorders.
METHODS
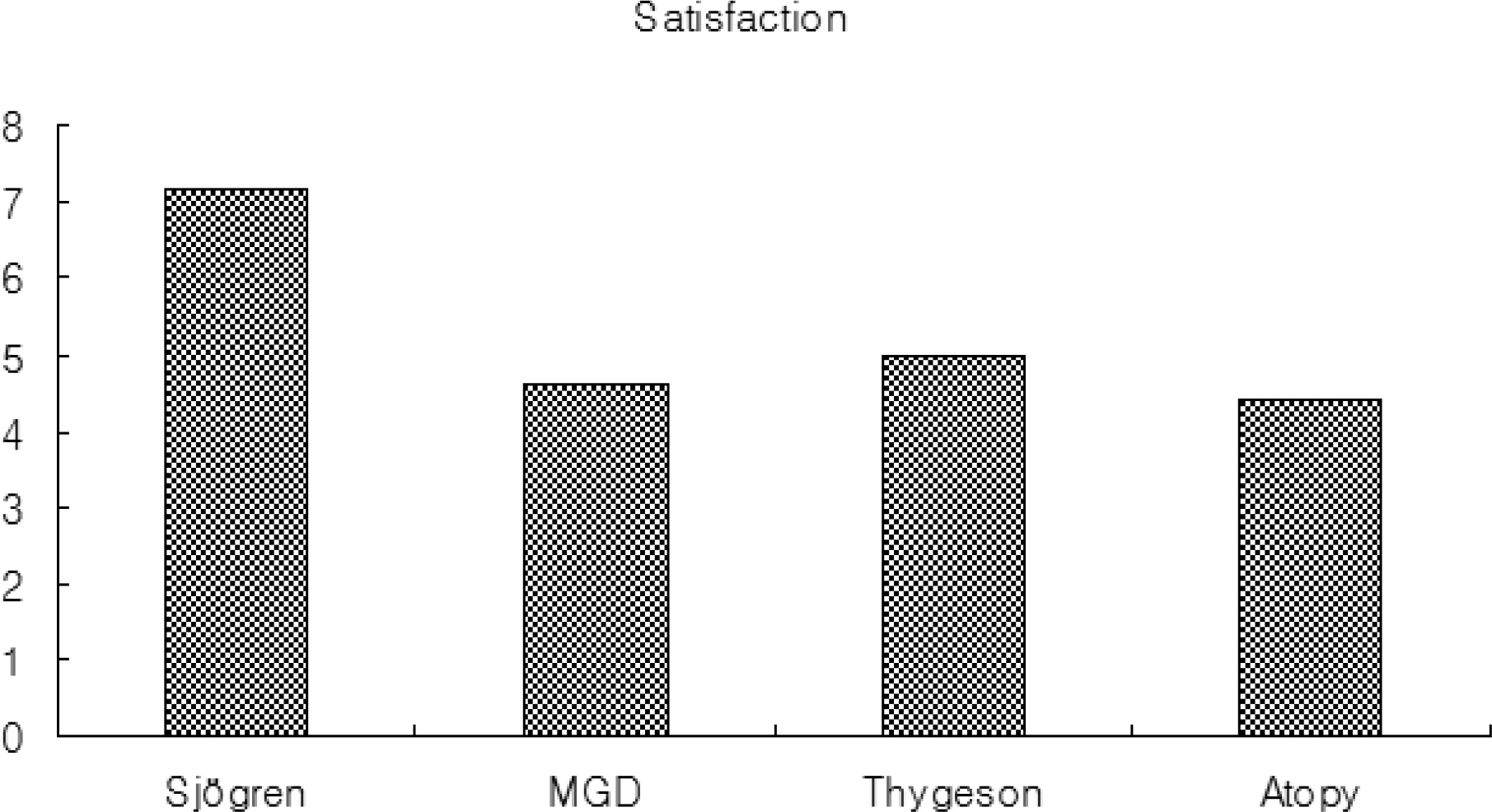
Thirty three patients with ocular surface diseases, including 17 with Sjogren syndrome, 8 with meibomian gland dysfunction (MGD), 4 with Thygeson's keratitis, and 4 with atopic keratoconjunctivitis (AKC) were treated with Restasis twice a day for 3 months. During follow up, the symptom severity assessment (burning, itching, foreign body sensation, blurring, photophobia, and pain), TBUT (tear break up time), Schirmer score, frequencies of artificial tear use, onset of symptomatic relief, subjective satisfaction score, and side effects were evaluated.
RESULTS
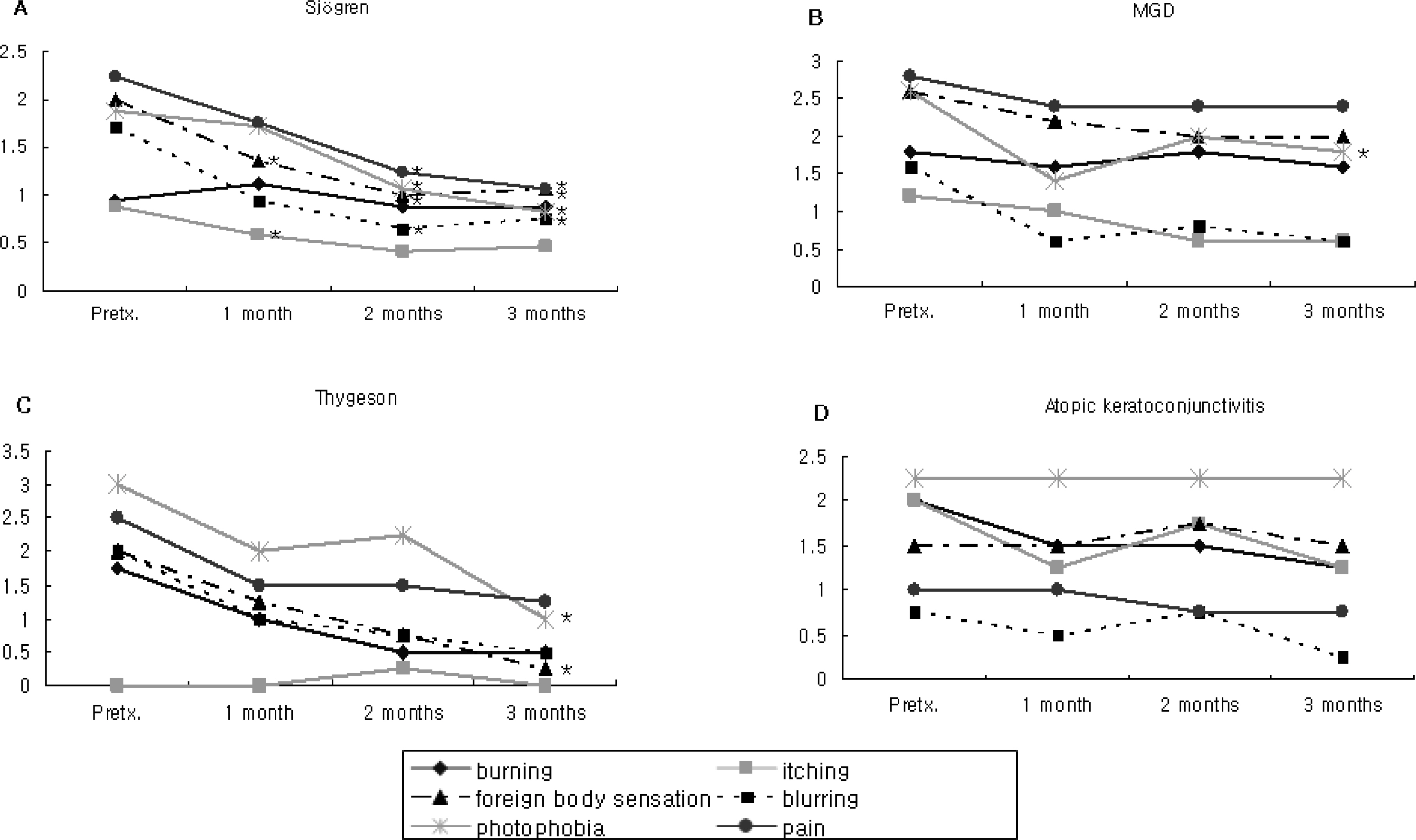
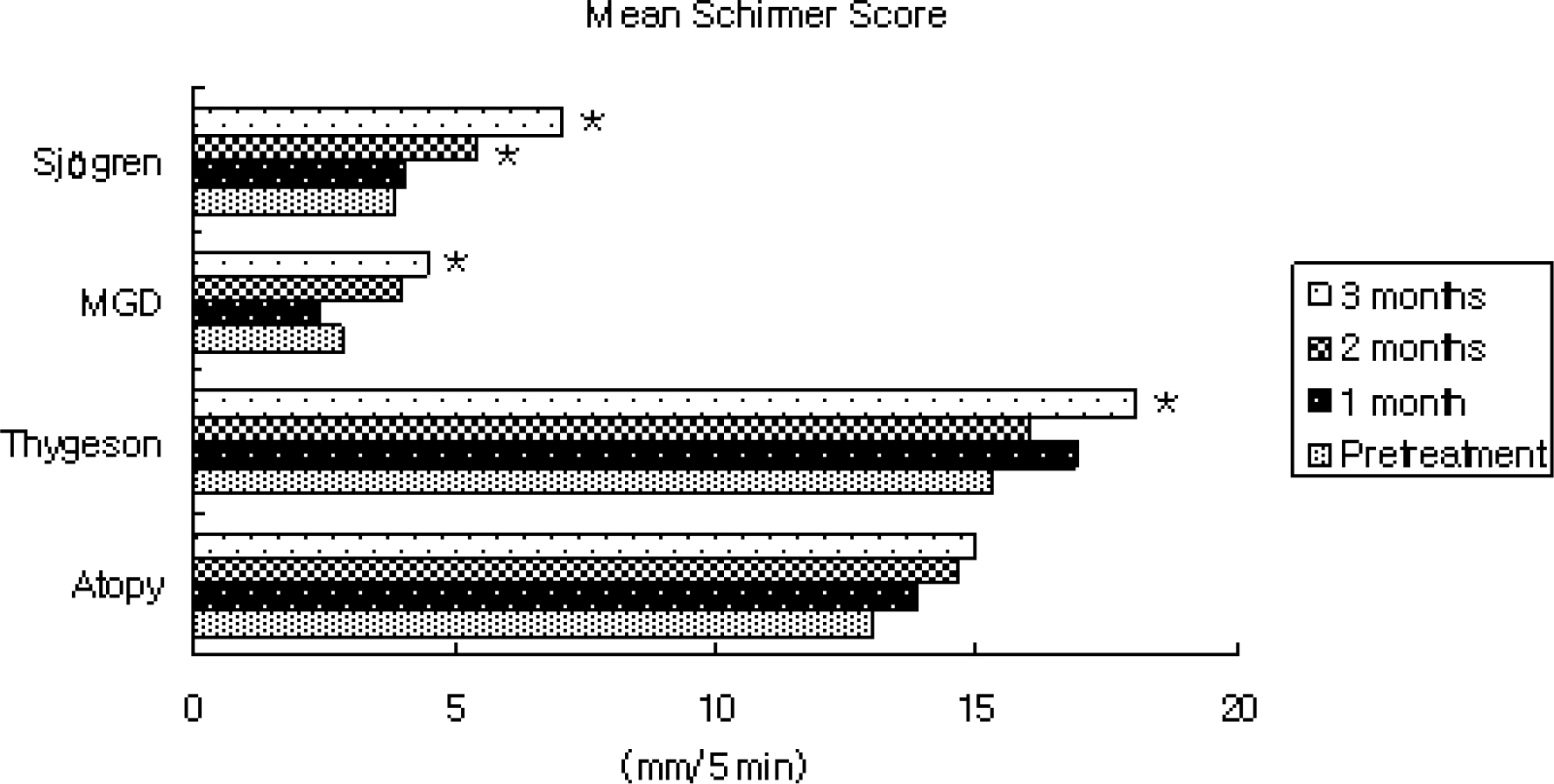
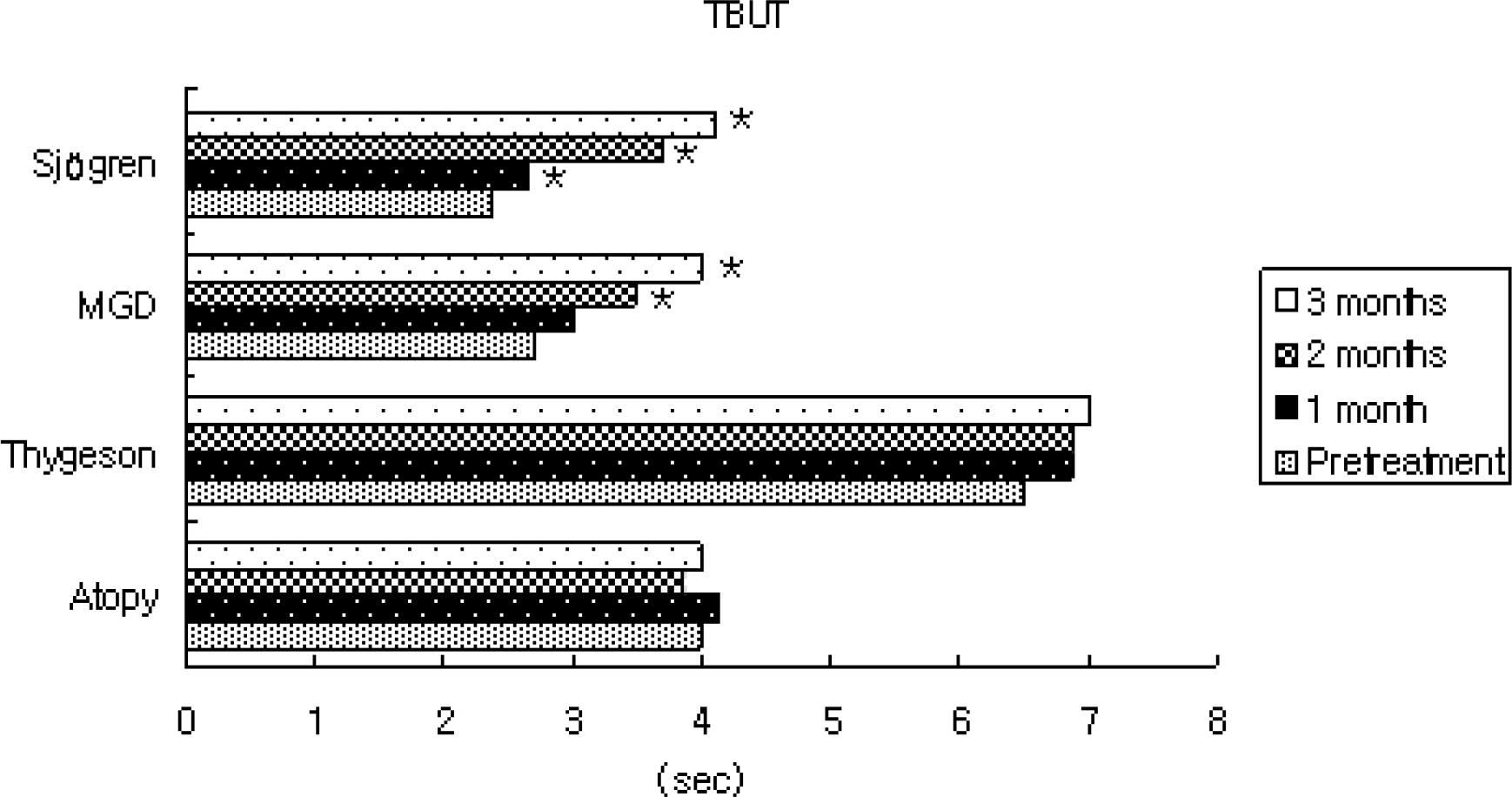
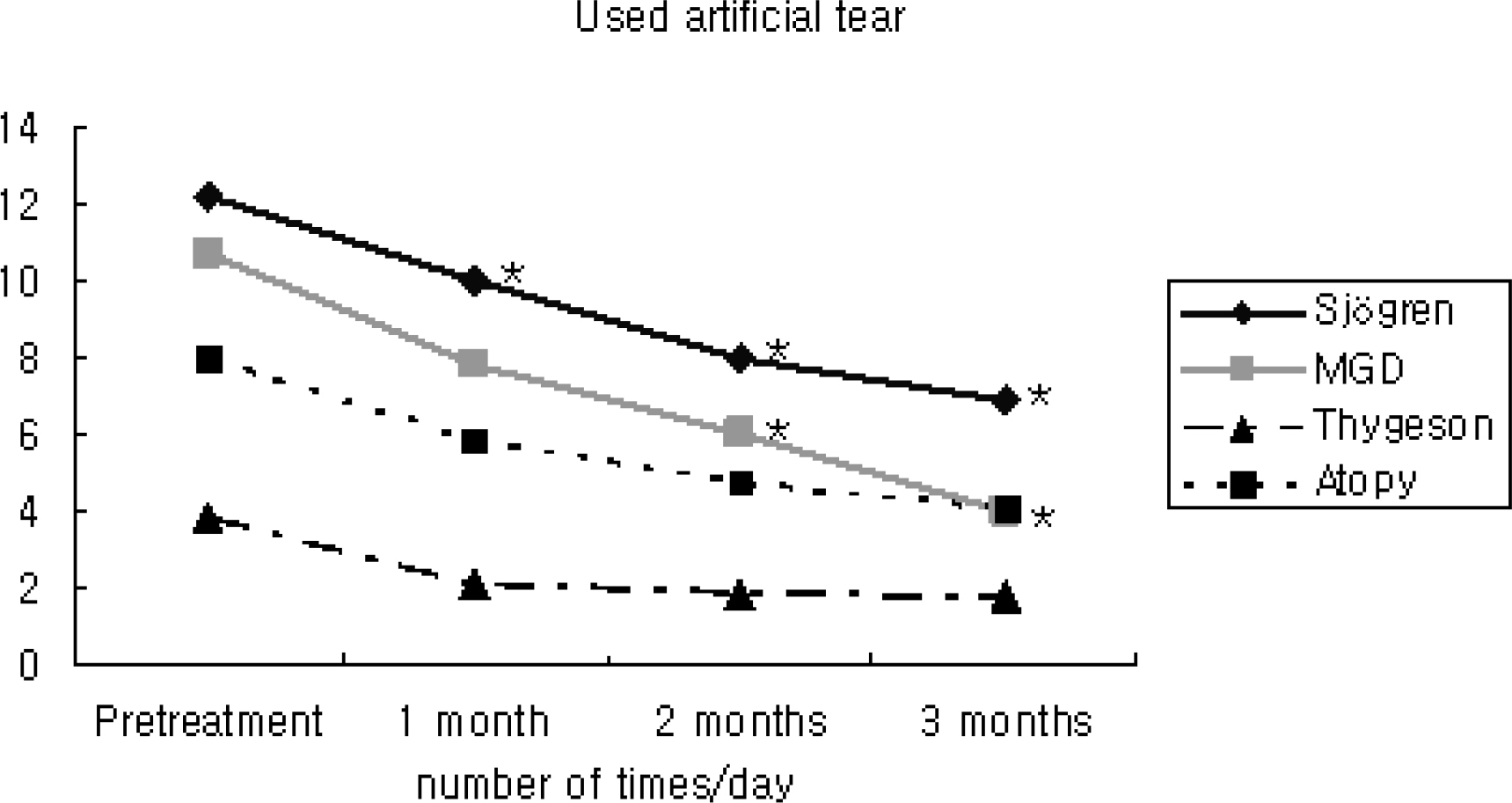
In patients with Sjogren syndrome, foreign body sensation, blurring, photophobia, and pain were reduced after treatment, and the mean Schirmer score, TBUT increased and frequencies of artificial tear use decreased significantly. In patients with MGD, photophobia was reduced after treatment, TBUT and artificial tear use improved after 2 months, and the Schirmer score increased at 3 months. In patient's with Thygeson's keratitis, foreign body sensation and photophobia reduced, and the Schirmer score was increased at 3 months. No significant changes in symptoms, Schirmer score, or TBUT were observed in patients with AKC. Of all subjects, 55% reported symptomatic relief between 3 and 5 weeks after treatment. The mean satisfaction score after treatment was the highest for patients with Sjogren syndrome. Two subjects reported a temporary burning sensation, and one subject quit using Restasis because of bitter taste and a burning sensation.
CONCLUSIONS
Treatment with Restasis appeared to be effective in treating dry eye symptoms in patients with Sjogren syndrome. It was shown to be partially helpful in patients with MGD and Thygeson's keratitis, while it showed no beneficial effect in patients with AKC.
MeSH Terms
Figure
Cited by 1 articles
-
Cinical Effect of Restasis® Eye Drops in Mild Dry Eye Syndrome
Jong Soo Lee, Tae Jin Yoon, Kyong Ho Kim
J Korean Ophthalmol Soc. 2009;50(10):1489-1494. doi: 10.3341/jkos.2009.50.10.1489.
Reference
-
References
1. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006; 25:900–7.2. Paik SH, Lee TS. The effect of Punctal Plug on Dry Eye Syndrome. J Korean Ophthalmol Soc. 1994; 35:737–44.3. Yi JY, Paik HJ. The clinical effect of the silicone intracanalicular plug in dry eye syndrome. J Korean Ophthalmol Soc. 1995; 36:1847–53.4. Avunduk AM, Avunduk MC, Varnell ED, Kaufman HE. The comparision of efficacies of topical corticosteroids and nonsteroidal anti– inflammatory drops on dry eye patients: A clinical and immunocytochemical study. Am J Ophthalmol. 2003; 136:593–602.5. Marsh P, Pflugfelder SC. Topical non– preserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren's syndrome. Ophthalmology. 1999; 106:811–6.6. Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004; 137:337–42.
Article7. O'Brien PD, Collum LM. Dry eye: diagnosis and current treatment strategies. Curr Allergy Asthma Rep. 2004; 43:314–9.8. Kunert KS, Tisdale AS, Stern ME, et al. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000; 118:1489–96.9. Perry HD, Donnenfeld ED. Topical 0.05% cyclosporine A in the treatment of dry eye. Expert Opin Pharmacother. 2004; 5:2099–107.10. Brignole F, Pisella P– J, de Saint Jean M, et al. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporine A. Invest Ophthamol Vis Sci. 2001; 42:90–5.11. Stonecipher K, Perry HD, Gross RH, Kerney DL. The impact of topical cyclosporine A emulsion 0.05% on the outcomes of patients with keratoconjunctivitis sicca. Curr Med Res Opin. 2005; 21:1057–63.
Article12. Barber LD, Pflugfelder SC, Tauber J, Foulks GN. Phase III safety evaluation of cyclosporine 0.1% ophthalmic emulsion administered twice daily to dry eye disease patients for up to 3 years. Ophthalmology. 2005; 112:1790–4.
Article13. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease [CsA Phase 3 Study Group]. Ophthalmology. 2000; 107:631–9.14. Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporine A ophthalmic emulsion in the treatment of moderate– to– severe dry eye disease: a dose– ranging randomized trial [The cyclosporon A phase 2 Study Group]. Ophthalmology. 2000; 107:967–74.15. Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocular Surface. 2004; 2:124–30.
Article16. Stern ME, Gao J, Siemasko KF, et al. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004; 78:409–16.
Article17. Dogru M, Tsubota K. New insights into the diagnosis and treatment of dry eye. Ocular Surface. 2004; 2:59–75.
Article18. Hong S, Kim T, Chung SH, et al. Recurrence after topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren's syndrome. J Ocul Phamacol Ther. 2007; 23:78–82.19. Driver PJ, Lemp MA. Meibomian gland dysfunction. Surv Ophthalmol. 1996; 40:343–67.
Article20. Perry HD, Doshi-Carnevale S, Donnefeld ED, et al. Efficacy of commercially available topical cyclosporine A 0.05% in the treatment of meibomian gland dysfunction. Cornea. 2006; 25:171–5.
Article21. Rubin M, Rao SN. Efficacy of topical cyclosporin 0.05% in the treatment of posterior blepharitis. J Ocul Pharmacol Ther. 2006; 22:47–53.
Article22. Hardten DR, Doughman DJ, Holland EJ, et al. Persistent superficial punctate keratitis after resolution of chlamydial follicular conjunctivitis. Cornea. 1992; 11:360–3.
Article23. Rdinhard T, Sundmacher R. Topical cyclosporin A in Thygeson's superficial punctate keratitis. Graefes Arch Clin Exp Ophthalmol. 1999; 237:109–12.24. Dogru M, Okada N, Asano-Kato N, et al. Alterations of the ocular surface epithelial mucins 1, 2, 4 and the tear functions in patients with atopic keratoconjunctivitis. Clin Exp Allergy. 2006; 36:1556–65.
Article25. Kilic A, Gurler B. Topical 2% cyclosporine A in preservative-free artificial tears for the treatment of vernal keratoconjunctivitis. Can J Ophthalmol. 2006; 41:693–8.26. Hingorani M, Moodaley L, Calder VL. et al. A randomized, placebo– controlled trial of topical cyclosporin A in steroid-dependent atopic keratoconjunctivitis. Ophthalmology. 1998; 105:1715–20.27. Tang– Liu DD, Acheampong A. Ocular pharmacokinetics and safety of cyclosporin, a novel topical treatment for dry eye. Clin Pharmacokinet. 2005; 44:247–61.28. Strong B, Farley W, Stern ME, Pflugfelder SC. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea. 2005; 24:80–5.
Article29. Akpek EK, Dart JK, Watson S, et al. A randomized trial of topical cyclosporin 0.05% in topical steroid– resistant atopic keratoconjunctivitis. Ophthalmology. 2004; 111:476–82.30. Daniell M, Constantinou M, Vu HT, Tralor HR. Randomised controlled trial of topical ciclosporin A in steroid dependent allergic conjunctivitis. Br J Ophthalmol. 2006; 90:461–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of 0.05% Cyclosporine A on the Ocular Surface after Photorefractive Keratectomy
- The Effect of Topical Cyclosporine 0.05% on Tear Osmolarity for Dry Eye Syndrome
- The Effect of Limbal Transplantation & Cyclosporine A for Chemically Damaged Rabbit Cornea
- Therapeutic Effects of 0.03% Tacrolimus Eye Drops for Chronic Ocular Graft-Versus-Host Disease
- Short Term Effects of Topical Cyclosporine and Viscoelastic on the Ocular Surfaces in Patients with Dry Eye