J Nutr Health.
2016 Apr;49(2):72-79. 10.4163/jnh.2016.49.2.72.
Elevated folic acid results in contrasting cancer cell line growth with implications for mandatory folic acid fortification
- Affiliations
-
- 1School of Biomedical Sciences and Pharmacy, University of Newcastle, Ourimbah NSW 2258, Australia.
- 2School of Environmental & Life Sciences, University of Newcastle, Ourimbah NSW 2258, Australia. 73805@ncc.re.kr
- 3Teaching & Research Unit, Central Coast Local Health District, Gosford NSW 2250, Australia.
- KMID: 2209844
- DOI: http://doi.org/10.4163/jnh.2016.49.2.72
Abstract
- PURPOSE
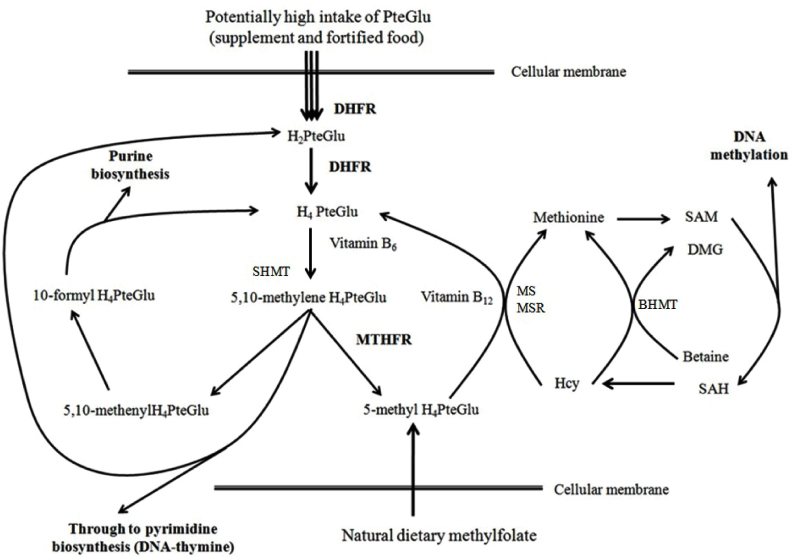
The initiation of mandatory folic acid fortification using pteroylmonoglutamic acid (PteGlu) has reduced the rate of congenital malformations. However, it also appears to be responsible for several adverse effects, including increased cancer incidence. This may be related to physicho-chemical characteristics of PteGlu. This study examines the potential effect of high concentrations of PteGlu on a population subjected to mandatory folic acid fortification using an in vitro model.
METHODS
Caco-2 (colorectal cancer) and MCF7 (breast cancer) cell lines were cultured at 6 different PteGlu concentrations (0, 0.1, 1, 50, 250, and 500µg/ml) for 6 days. Cell growth was determined using thiazolyl blue tetrazolium bromide assay. The genotype of dihydrofolate reductase 19bp deletion/insertion (DHFR 19-del) was also scored in cell lines using a restriction fragment length polymorphism technique to examine whether genetic variations may factor in cell proliferation.
RESULTS
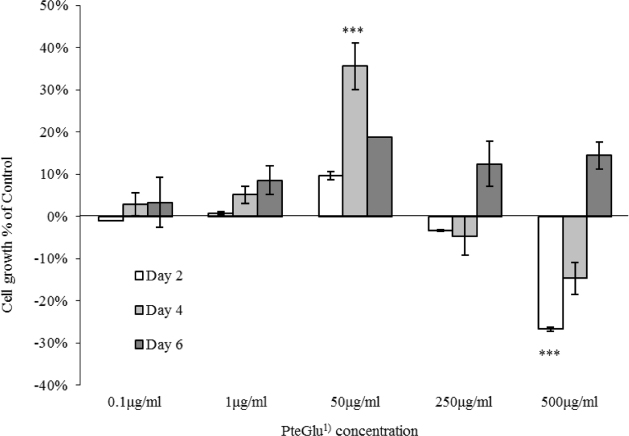
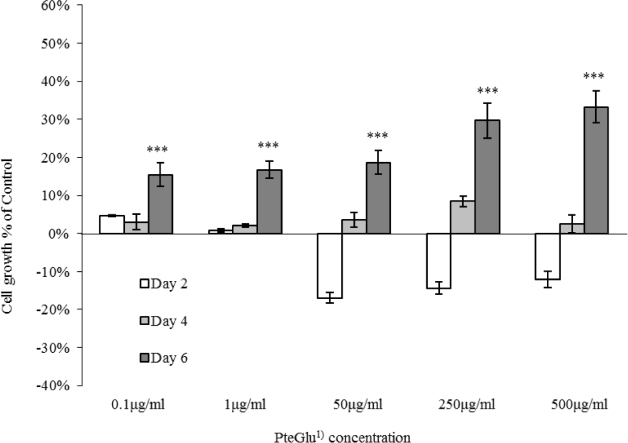
PteGlu exhibited differential growth promoting properties between cell lines. Caco-2 cells did not show a significant growth difference at low concentrations compared to control, however, at higher concentrations, the growth showed a contrasting trend in the early experimental period, while MCF7 showed enhanced cell growth at all concentrations. The DHFR 19-del genotype differed in the two cell lines.
CONCLUSION
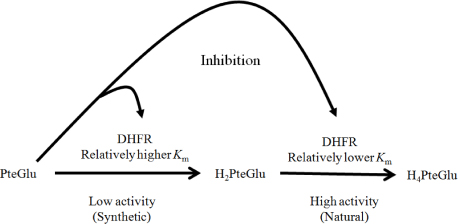
Altered response to PteGlu by Caco-2 and MCF7 may reflect a tissue specific disease aetiology or genotype specific differential enzyme activity, for example by DHFR, to critical levels of PteGlu. As folic acid fortification is a blanket intervention, and DHFR and other enzyme activities vary between individuals, PteGlu intake may have an as yet undefined effect on health. These findings may be relevant when considering mandatory folic acid fortification for disease prevention.
Keyword
MeSH Terms
Figure
Reference
-
1. Prinz-Langenohl R, Fohr I, Pietrzik K. Beneficial role for folate in the prevention of colorectal and breast cancer. Eur J Nutr. 2001; 40(3):98–105.
Article2. Kim YI. Folate and colorectal cancer: an evidence-based critical review. Mol Nutr Food Res. 2007; 51(3):267–292.
Article3. Kim YI. Role of folate in colon cancer development and progression. J Nutr. 2003; 133:11 Suppl 1. 3731S–3739S.
Article4. Kim YI. Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem. 1999; 10(2):66–88.
Article5. Choi SW, Mason JB. Folate status: effects on pathways of colorectal carcinogenesis. J Nutr. 2002; 132:8 Suppl. 2413S–2418S.
Article6. Bills ND, Hinrichs SH, Morgan R, Clifford AJ. Delayed tumor onset in transgenic mice fed a low-folate diet. J Natl Cancer Inst. 1992; 84(5):332–337.
Article7. Van Guelpen B, Hultdin J, Johansson I, Hallmans G, Stenling R, Riboli E, Winkvist A, Palmqvist R. Low folate levels may protect against colorectal cancer. Gut. 2006; 55(10):1461–1466.
Article8. Stolzenberg-Solomon RZ, Chang SC, Leitzmann MF, Johnson KA, Johnson C, Buys SS, Hoover RN, Ziegler RG. Folate intake, alcohol use, and postmenopausal breast cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr. 2006; 83(4):895–904.
Article9. Lucock M, Yates Z. Folic acid fortification: a double-edged sword. Curr Opin Clin Nutr Metab Care. 2009; 12(6):555–564.
Article10. Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CA, Snover DC, Church TR, Allen JI, Robertson DJ, Beck GJ, Bond JH, Byers T, Mandel JS, Mott LA, Pearson LH, Barry EL, Rees JR, Marcon N, Saibil F, Ueland PM, Greenberg ER. Polyp Prevention Study Group. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007; 297(21):2351–2359.11. Baggott JE, Morgan SL. Folic acid supplements are good (not bad) for rheumatoid arthritis patients treated with low-dose methotrexate. Am J Clin Nutr. 2008; 88(2):479–480.
Article12. Ebbing M, Bønaa KH, Nygård O, Arnesen E, Ueland PM, Nordrehaug JE, Rasmussen K, Njølstad I, Refsum H, Nilsen DW, Tverdal A, Meyer K, Vollset SE. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009; 302(19):2119–2126.13. Lajous M, Romieu I, Sabia S, Boutron-Ruault MC, Clavel-Chapelon F. Folate, vitamin B12 and postmenopausal breast cancer in a prospective study of French women. Cancer Causes Control. 2006; 17(9):1209–1213.
Article14. Ericson U, Sonestedt E, Gullberg B, Olsson H, Wirfält E. High folate intake is associated with lower breast cancer incidence in postmenopausal women in the Malm Diet and Cancer cohort. Am J Clin Nutr. 2007; 86(2):434–443.
Article15. Lin J, Lee IM, Song Y, Cook NR, Selhub J, Manson JE, Buring JE, Zhang SM. Plasma homocysteine and cysteine and risk of breast cancer in women. Cancer Res. 2010; 70(6):2397–2405.
Article16. Larsson SC, Giovannucci E, Wolk A. Folate and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2007; 99(1):64–76.
Article17. Stevens VL, McCullough ML, Sun J, Gapstur SM. Folate and other one-carbon metabolism-related nutrients and risk of postmenopausal breast cancer in the Cancer Prevention Study II Nutrition Cohort. Am J Clin Nutr. 2010; 91(6):1708–1715.
Article18. Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999-2000. Am J Clin Nutr. 2005; 82(2):442–450.
Article19. Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001; 285(23):2981–2986.
Article20. Kalmbach RD, Choumenkovitch SF, Troen AM, D'Agostino R, Jacques PF, Selhub J. Circulating folic acid in plasma: relation to folic acid fortification. Am J Clin Nutr. 2008; 88(3):763–768.
Article21. Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, Selhub J, McTiernan A, Yasui Y, Oral E, Potter JD, Ulrich CM. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006; 136(1):189–194.
Article22. Waller DK, Tita AT, Annegers JF. Rates of twinning before and after fortification of foods in the US with folic acid, Texas, 1996 to 1998. Paediatr Perinat Epidemiol. 2003; 17(4):378–383.
Article23. Arabelovic S, Sam G, Dallal GE, Jacques PF, Selhub J, Rosenberg IH, Roubenoff R. Preliminary evidence shows that folic acid fortification of the food supply is associated with higher methotrexate dosing in patients with rheumatoid arthritis. J Am Coll Nutr. 2007; 26(5):453–455.
Article24. Hirsch S, Sanchez H, Albala C, de la Maza MP, Barrera G, Leiva L, Bunout D. Colon cancer in Chile before and after the start of the flour fortification program with folic acid. Eur J Gastroenterol Hepatol. 2009; 21(4):436–439.
Article25. Mason JB, Dickstein A, Jacques PF, Haggarty P, Selhub J, Dallal G, Rosenberg IH. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2007; 16(7):1325–1329.
Article26. Lucock M, Ng X, Boyd L, Skinner V, Wai R, Tang S, Naylor C, Yates Z, Choi JH, Roach P, Veysey M. TAS2R38 bitter taste genetics, dietary vitamin C, and both natural and synthetic dietary folic acid predict folate status, a key micronutrient in the pathoaetiology of adenomatous polyps. Food Funct. 2011; 2(8):457–465.
Article27. Novakovic P, Stempak JM, Sohn KJ, Kim YI. Effects of folate deficiency on gene expression in the apoptosis and cancer pathways in colon cancer cells. Carcinogenesis. 2006; 27(5):916–924.
Article28. Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988; 48(3):589–601.29. Johnson WG, Stenroos ES, Spychala JR, Chatkupt S, Ming SX, Buyske S. New 19 bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR): a risk factor for spina bifida acting in mothers during pregnancy? Am J Med Genet A. 2004; 124A(4):339–345.
Article30. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991; 338(8760):131–137.31. Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, Mulinare J, Zhao P, Wong LY, Gindler J, Hong SX, Correa A. China-U.S. Collaborative Project for Neural Tube Defect Prevention. Prevention of neural-tube defects with folic acid in China. N Engl J Med. 1999; 341(20):1485–1490.
Article32. Yang QH, Botto LD, Gallagher M, Friedman JM, Sanders CL, Koontz D, Nikolova S, Erickson JD, Steinberg K. Prevalence and effects of gene-gene and gene-nutrient interactions on serum folate and serum total homocysteine concentrations in the United States: findings from the third National Health and Nutrition Examination Survey DNA Bank. Am J Clin Nutr. 2008; 88(1):232–246.
Article33. Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. 2008; 87(3):517–533.
Article34. Matthews RG, Haywood BJ. Inhibition of pig liver methylenetetrahydrofolate reductase by dihydrofolate: some mechanistic and regulatory implications. Biochemistry. 1979; 18(22):4845–4851.
Article35. Bailey SW, Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci U S A. 2009; 106(36):15424–15429.
Article36. Chen MJ, Shimada T, Moulton AD, Cline A, Humphries RK, Maizel J, Nienhuis AW. The functional human dihydrofolate reductase gene. J Biol Chem. 1984; 259(6):3933–3943.
Article37. Albrecht AM, Biedler JL, Hutchison DJ. Two different species of dihydrofolate reductase in mammalian cells differentially resistant to amethopterin and methasquin. Cancer Res. 1972; 32(7):1539–1546.38. Goto Y, Yue L, Yokoi A, Nishimura R, Uehara T, Koizumi S, Saikawa Y. A novel single-nucleotide polymorphism in the 3-untranslated region of the human dihydrofolate reductase gene with enhanced expression. Clin Cancer Res. 2001; 7(7):1952–1956.39. Parle-McDermott A, Pangilinan F, Mills JL, Kirke PN, Gibney ER, Troendle J, O'Leary VB, Molloy AM, Conley M, Scott JM, Brody LC. The 19-bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR) may decrease rather than increase risk for spina bifida in the Irish population. Am J Med Genet A. 2007; 143A(11):1174–1180.
Article40. Xu X, Gammon MD, Wetmur JG, Rao M, Gaudet MM, Teitelbaum SL, Britton JA, Neugut AI, Santella RM, Chen J. A functional 19-base pair deletion polymorphism of dihydrofolate reductase (DHFR) and risk of breast cancer in multivitamin users. Am J Clin Nutr. 2007; 85(4):1098–1102.
Article41. Lucock M, Yates Z. Folic acid - vitamin and panacea or genetic time bomb? Nat Rev Genet. 2005; 6(3):235–240.
Article42. Junaid MA, Kuizon S, Cardona J, Azher T, Murakami N, Pullarkat RK, Brown WT. Folic acid supplementation dysregulates gene expression in lymphoblastoid cells--implications in nutrition. Biochem Biophys Res Commun. 2011; 412(4):688–692.43. Kelly P, McPartlin J, Goggins M, Weir DG, Scott JM. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. Am J Clin Nutr. 1997; 65(6):1790–1795.
Article44. Assaraf YG. Molecular basis of antifolate resistance. Cancer Metastasis Rev. 2007; 26(1):153–181.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of polyneuropathy associated with folic acid deficiency
- Folate during reproduction: the Canadian experience with folic acid fortification
- Folic acid metabolism and the side effect of the methotrexate in rheumatoid arthritis
- Influence of folic acid knowledge on effective folic acid intake in Chinese pregnant women: a cross-sectional study
- Serum levels of folic acid and vitamin B12 in Korean patients with vitiligo