J Lung Cancer.
2006 Dec;5(2):75-83. 10.6058/jlc.2006.5.2.75.
Clinical Effectiveness of Tumor Markers (CEA, NSE, Cyfra 21-1) in Completely Resected Non-small Cell Lung Cancer
- Affiliations
-
- 1Department of Thoracic and Cardiovascular Surgery, Yongdong Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. dylee@yumc.yonsei.ac.kr
- 2Department of Thoracic and Cardiovascular Surgery, Eulji University Hospital, Eulji University, Seoul, Korea.
- 3Department of Pathology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2200165
- DOI: http://doi.org/10.6058/jlc.2006.5.2.75
Abstract
-
PURPOSE: The applicability of tumor markers still remains controversial in non-small cell lung cancer (NSCLC) due to lower sensitivity & specificity. And, tumor markers actually have not been used determining treatment plans in NSCLC patients yet. So, we evaluated correlation between levels of serum tumor marker (CEA, NSE and Cyfra 21-1) and prognosis in NSCLC patients underwent complete surgical resection.
MATERIALS AND METHODS
We retrospectively studied 64 NSCLC patients underwent complete surgical resection in Yongdong severance hospital from April 2002 to October 2005. Preoperative and postoperative serum levels of tumor markers (CEA, NSE, Cyfra 21-1) were measured with commercialized kits and the correlation between the serum levels of tumor markers and prognosis was evaluated. Normal cutoff values of CEA, NSE and Cyfra 21-1 were 5.0 ng/ml, 12.5 ng/ml and 3.2 ng/ml. We estimated recurrence or distant metastasis with computed tomography, magnetic resonance imaging, whole body bone scan, positron emission tomography and biopsy.
RESULTS
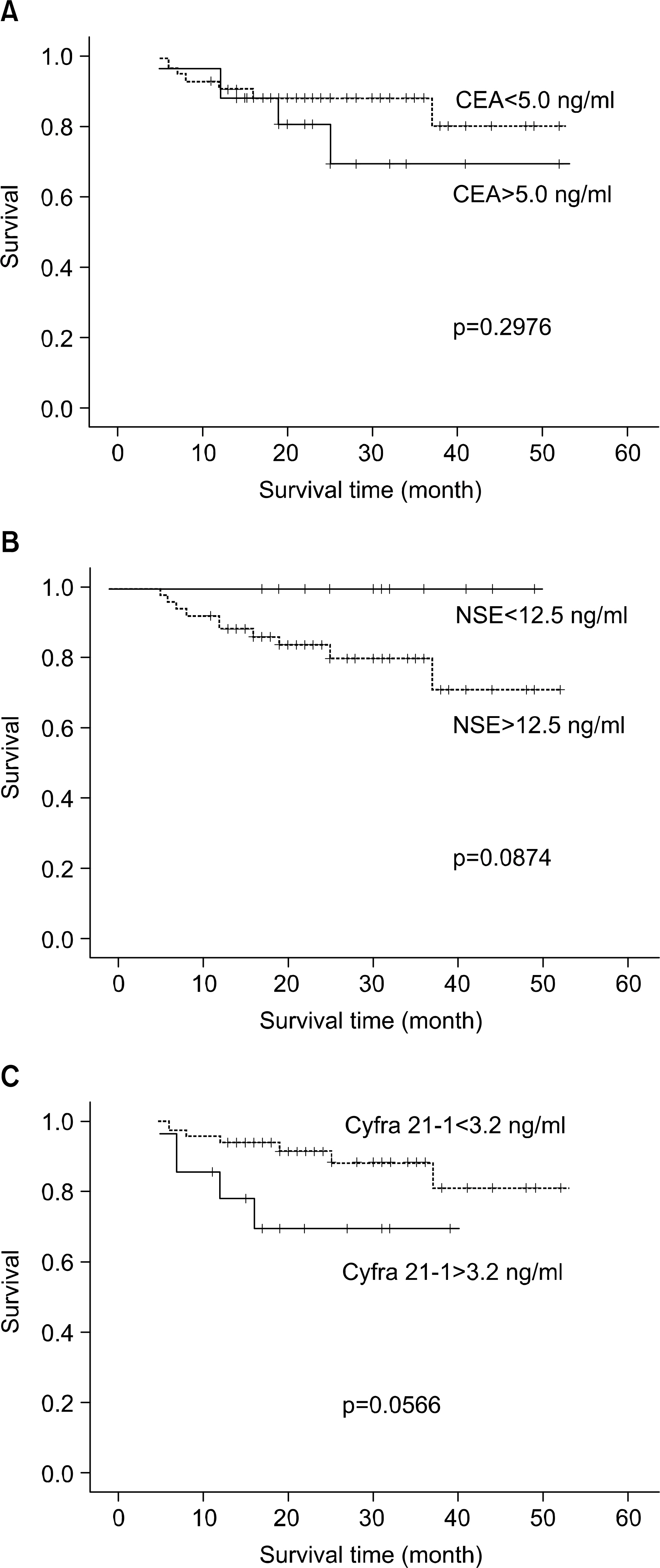
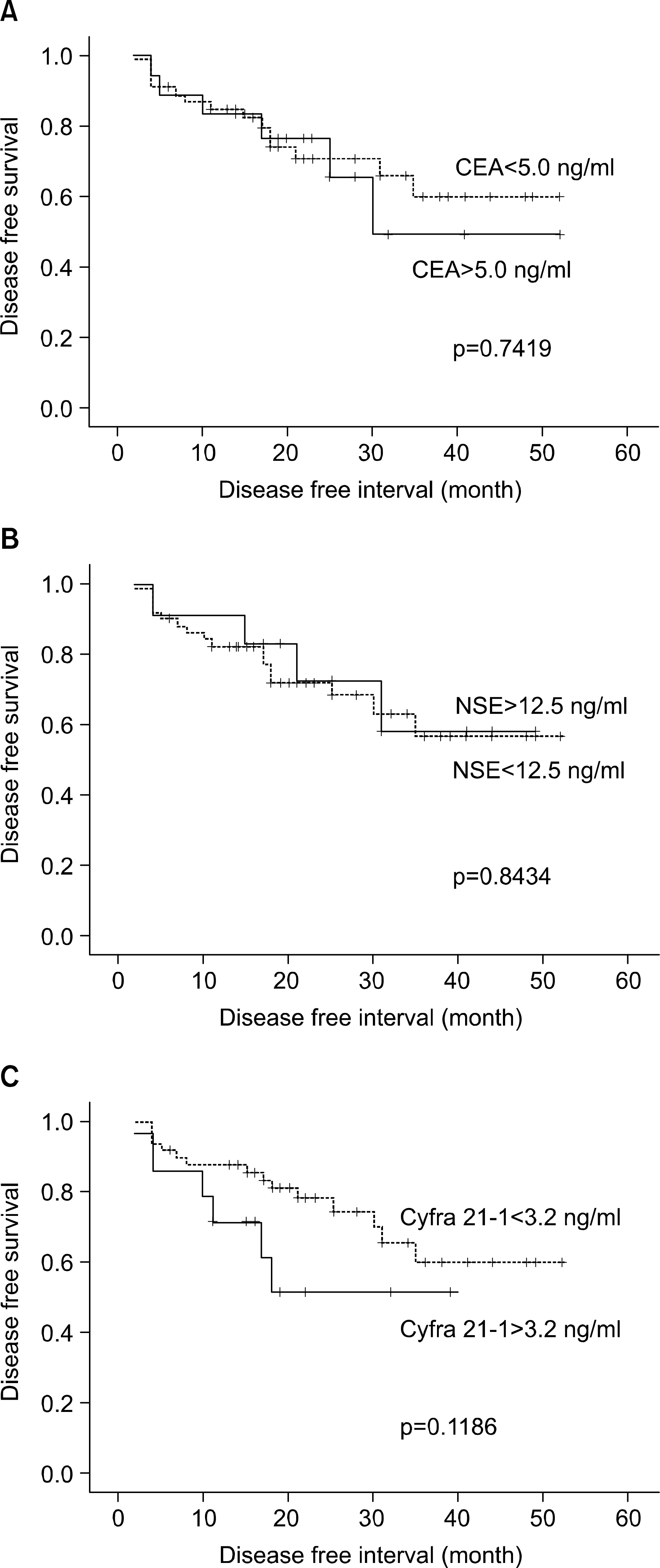
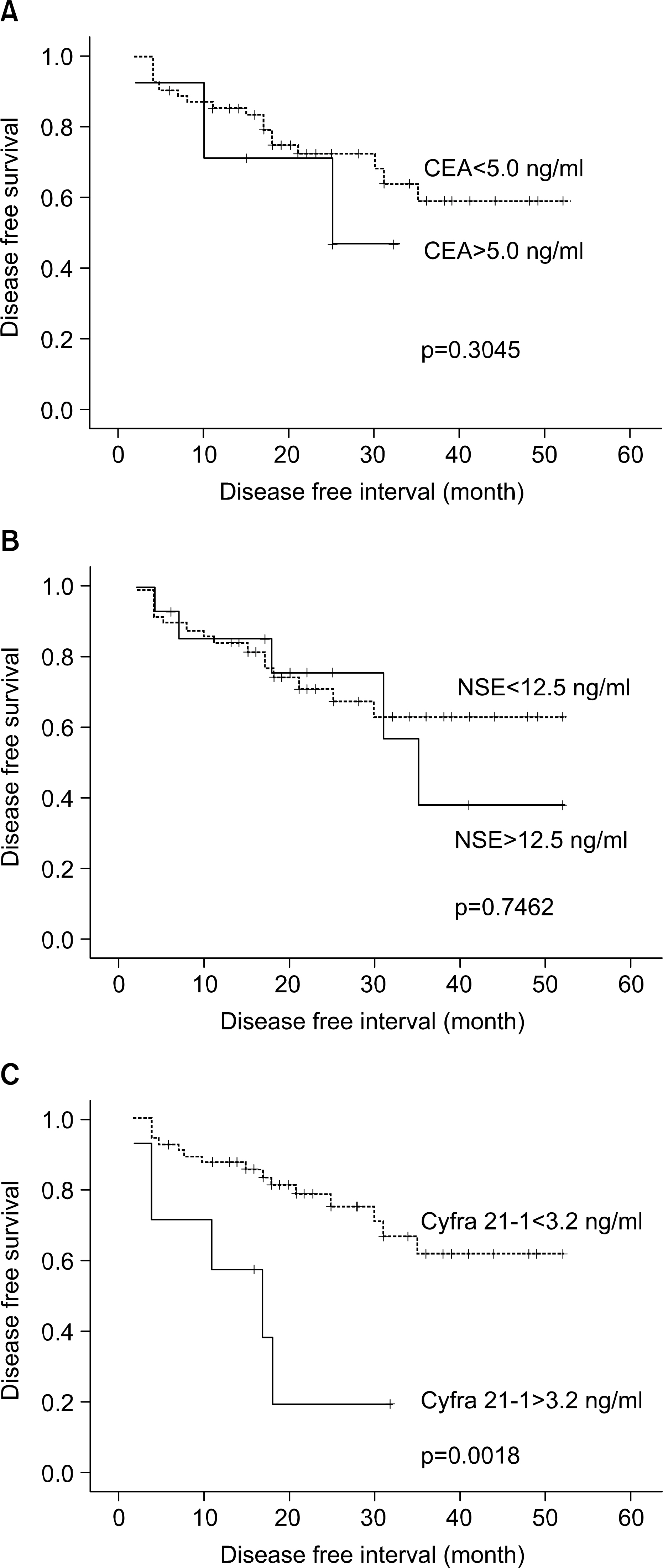
Preoperative and postoperative serum levels of tumor markers were not significantly correlated with lung cancer stages and histologies. The elevated levels of postoperative CEA (p=0.0142) and Cyfra 21-1 (p=0.0105) were correlated with shortened survival time. And, the shortened disease free interval was significantly associated with the elevated level of postoperative Cyfra 21-1 (p=0.0018). The elevated level of preoperative Cyfra 21-1 (p=0.0566) had a tendency to relate the shortened survival time, but it didn't reach statistical importance.
CONCLUSION
Considering previous results, especially Cyfra 21-1 may be useful prognostic factor in predicting survival times, and recurrence or metastasis. But, further study and longer follow-up period were needed to make conclusion regarding usefulness of other tumor markers
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Diagnostic Utility of Serum Cytokeratin Fragment 21-1 in Patients with Lung Cancer
Sungwook Song, Eun-Hyung Yoo, Hyun-Jung Cho
Lab Med Online. 2015;5(3):143-148. doi: 10.3343/lmo.2015.5.3.143.Biomarkers for Lung Cancer
Sei Hoon Yang
J Lung Cancer. 2009;8(2):67-77. doi: 10.6058/jlc.2009.8.2.67.Serum Carcinoembryonic Antigen as an Index of the Therapeutic Effect of EGFR-TKIs in Patients with Advanced Non-Small Cell Lung Cancer
Jin Hee Park, Sung Bin Kim, Sung Jin Nam, Su Hyeon Jeong, Chul Ho Oak, Tae Won Jang, Maan Hong Jung
J Lung Cancer. 2010;9(2):97-102. doi: 10.6058/jlc.2010.9.2.97.
Reference
-
1.Fu XL., Zhu XZ., Shi DR, et al. Study of prognostic predictors for non-small cell lung cancer. Lung Cancer. 1999. 23:143–152.
Article2.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997. 111:1710–1717.
Article3.van der Gaast A., Schoenmakers CH: Kok TC: Blijenberg BG: Comille F., Splinter TA. Evaluation of a new tumour marker in patients with non-small-cell lung cancer: Cyfra 21.1. Br J Cancer. 1994. 69:525–528.4.Diez M: Torres A: Maestro ML, et al. Prediction of survival and recurrence by serum and cytosolic levels of CEA: CA125 and SCC antigens in resectable non-small-cell lung cancer. Br J Cancer. 1996. 73:1248–1254.
Article5.Lombardi C., Tassi GF., Pizzocolo G., Donato F. Clinical significance of a multiple biomarker assay in patients with lung cancer. A study with logistic regression analysis. Chest. 1990. 97:639–644.6.Vincent RG., Chu TM., Fergen TB: Ostrander M. Carcino-embryonic antigen in 228 patients with carcinoma of the lung. Cancer. 1975. 36:2069–2076.
Article7.Shinkai T., Saijo N: Tominaga K, et al. Serial plasma carcinoembryonic antigen measurement for monitoring patients with advanced lung cancer during chemotherapy. Cancer. 1986. 57:1318–1323.
Article8.Nisman B: Amir G: Lafair J, et al. Prognostic valvue of Cyfra 21-1: TPS and CEA in different histologic types of non-small cell lung cancer. Anticancer Res. 1999. 19:3549–3552.9.American Thoracic Society and European Respiratory Society. Pretreatment evaluation of non-small cell lung cancer. Am Rev Rcspir Crit Care Med. 1997. 156:320–332.10.Jorgensen LGM., Hansen HHH., Cooper EH. Neuron specific enolase: carcino-embryonic antigen and lactate dehydrogenase as indicators of disease activity in small lung cancer. Eur J Cancer Clin Oncol. 1989. 25:123–128.11.Diez M., Torres A., Ortega L, et al. Value of serum neuronspecific enolase in nonsmall cell lung cancer. Oncology. 1993. 50:127–131.
Article12.Van Zandwijk N., Jssem E., Bonfrer JMG: Mooi WJ: van Tinteren H. Serum neuron-specific enolase and lactate dehydrogenase as predictors of response to chemotherapy and survival in non-small cell lung cancer. Semin Oncol. 1992. 19:37–43.13.Pujol JL: Boher JM: Grenier J., Quantin X. Cyfra 21-1: neuron specific enolase and prognosis of non-small cell lung cancer: prospective study in 62! patients. Lung Cancer. 2001. 31:221–231.14.Molina R., Filella X: Auge JM, et al. Tumor markers (CEA, CA 125, Cyfra 21-1: SCC and NSE) in patients with non-small cell 】ung cancer as an aid in histological diagnosis and progntysis. Tumor Biol. 2003. 24:209–218.15.Moll R: Franke WW., Schiller DL., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982. 31:11–24.
Article16.Stieber P., Hasholzner U., Bodenmuller H, et al. Cyfra 21-1. A new marker in lung cancer. Cancer. 1993. 72:707–713.
Article17.Nisman B., Lafair J., Heching N, et al. Evaluation of tissue polypeptide specific antigen, Cyfra 21-1, and carcinoembryonic antigen in nonsmall cell lung carcinoma: does the combined use of cytokeratin markers give any additional information? Cancer. 1998. 82:1850–1859.18.Pujol JL., Grenier J., Parrat E, et al. Cytokeratins as serum markers in lung cancena comparison of Cyfra 21-1 and TPS. Am J Respir Crit Care Med. 1996. 154:725–733.19.Szturmowicz M., Sakowicz A., Rudzinski P, et al. The clinical value of Cyfra 21-1 estimation of lung cancer patients. Int J Biol Markers. 1996. 11:172–177.20.Travis WD: Brambilla E: Muller-Hermelink HK: Harris CC. The World Health Organization Classification of Tumours. Tumours of (he lung, pleura, thymus and heart. 1st ed.Lyon: IARC Press;2004. p. 10–11.21.Fletcher RH. Carcinoembryonic antigen. Ann Intern Med. 1986. 104:66–73.
Article22.Noriyoshi S., Mitsunori 0: Shin-ichi T, et al. Serum carcinoembryonic antigen level in surgical resected clinical stage I patients with non-small cell lung cancer. Ann Thoracic Surg. 2002. 74:174–179.23.Stokes TC., Stevens JF., Long P., Lockey E., Miller AL. Preoperative carcinoembryonic antigen and survival after resection of lung cancer. Br J Dis Chest. 1980. 74:390–394.
Article24.Dent PB., McCulloch PB., Wesley-James 0: MacLaren R., Muirhead W., Dunnett CW. Measurement of carcinoembryonic antigen in patients with bronchogenic carcinoma. Cancer. 1978. 42:1484–1491.
Article25.Klesibauer JP., Castelnau O., Thomas P., Ramirez J: Lanteaume A: Roux F. Prognostic value of pre-therapeutic levels of carcino-embryonic antigen in primary bronchial carcinoma. Bull Cancer. 1995. 82:1019–1024.26.Sawabata N., Ohta M., Takeda S, et al. Serum carcinoembryonic antigen level in surgically resected clinical stage I patients with non-small cell lung cancer. Ann Thorac Surg. 2002. 74:174–179.
Article27.Jan K., Wojcik E., Reinfuss M., Kolodziejski L. Carcinoembryonic antigen» squamous cell carcinoma antigen, Cyfra 21-1: and neuro-specific enolase in squamous cell lung cancer patients. Clin Chem. 2002. 48:1931–1937.28.Brechot JM., Chevret S., Nataf J, et al. Diagnostic and prognostic value of Cyfra 21-1 compared with other tumour markers in patients with non-small cell lung cancer: a prospective study of 116 patients. Eur J Cancer. 1997. 33:385–391.29.Barlesi F: Gimenez C., Torre JP, et al. Prognostic value of combination of Cyfra 21-1, CEA and NSE in patients with advanced non-small cell lung cancer. Res Med. 2004. 98:357–362.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CYFRA 21-1, The New Marker for Lung Cancer
- Usefulness of cyfra 21-1 as a tumor marker of lung cancer
- Performances of CYFRA 21-1, Carcinoembryonic Antigen and Their Combination for Lung Cancer Diagnosis
- Diagnostic Usefulness of Simultaneous Measurement ofSerum Tumor Markers in Lung Cancer Patients
- Pathologic Correlation of Serum Carcinoembryonic Antigen and Cytokeratin 19 Fragment in Resected Nonsmall Cell Lung Cancer