J Korean Soc Hypertens.
2012 Dec;18(4):146-153. 10.5646/jksh.2012.18.4.146.
Altered Regulation of Endothelin, Atrial Natriuretic Peptide, and Nitric Oxide Systems in Angiotensin II-induced Hypertension
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea. skimw@chonnam.ac.kr
- 2Department of Physiology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2196541
- DOI: http://doi.org/10.5646/jksh.2012.18.4.146
Abstract
- BACKGROUND
The present study aimed to determine the changes of endothelin (ET), nitric oxide, and atrial natriuretic peptide (ANP) systems in the kidney and aorta in angiotensin (Ang) II-induced hypertension.
METHODS
Male Sprague-Dawley rats were used. Ang II (100 ng.min-1.kg-1) was infused through entire time course. Fourteen days after beginning the regimen, aorta and kidney were taken. The protein expression of nitroc oxide synthase (NOS) was determined by semiquantitative immunoblotting. The mRNA expression of components of ET, NOS, ANP system was determined by real-time polymerase chain reaction.
RESULTS
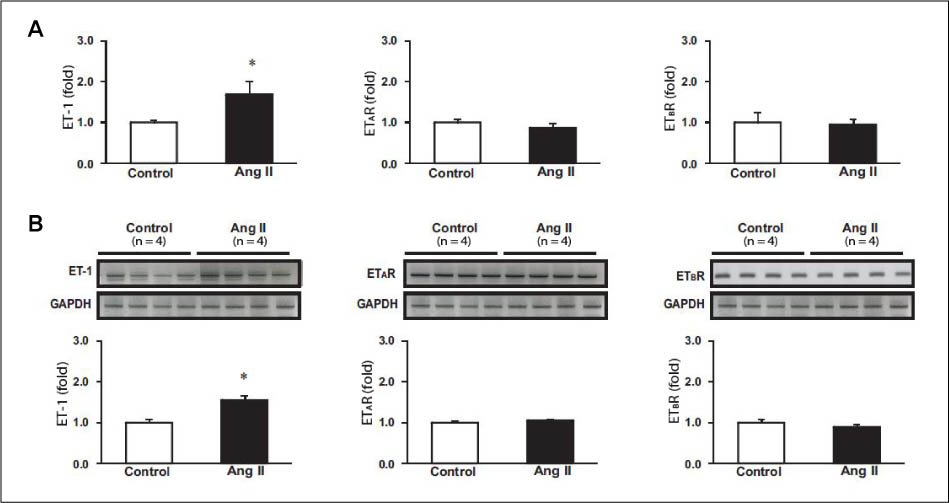
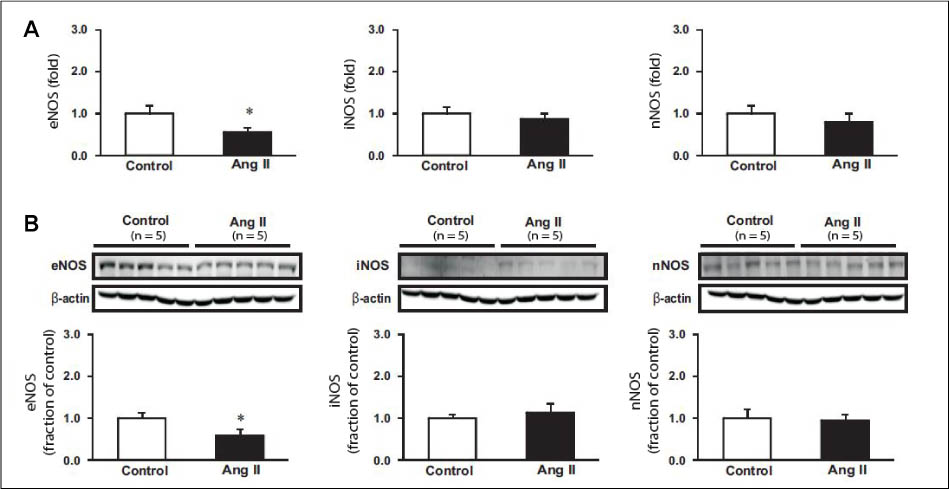
Hypertension was developed in the experimental group. mRNA expression of ET-1 in the aorta and kidney was increased. The protein expression of endothelial NOS (eNOS) was decreased in the aorta, while that of inducible NOS and neuronal NOS remained unaltered. mRNA expression of ANP, natriuretic peptide type (NPR)-A, and NPR-C was not changed in the aorta.
CONCLUSIONS
Based on these results, it seems that in Ang II-induced hypertensive rats, increased expression of ET-1 in the aorta and kidney, and decreased eNOS expression in the aorta contribute to the pathogenesis of hypertension.
MeSH Terms
Figure
Reference
-
1. Chin SY, Wang CT, Majid DS, Navar LG. Renoprotective effects of nitric oxide in angiotensin II-induced hypertension in the rat. Am J Physiol. 1998. 274(5 Pt 2):F876–F882.2. Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, et al. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004. 44:223–229.
Article3. Miller RC, Pelton JT, Huggins JP. Endothelins: from receptors to medicine. Trends Pharmacol Sci. 1993. 14:54–60.4. Benigni A, Zola C, Corna D, Orisio S, Facchinetti D, Benati L, et al. Blocking both type A and B endothelin receptors in the kidney attenuates renal injury and prolongs survival in rats with remnant kidney. Am J Kidney Dis. 1996. 27:416–423.
Article5. Sigmon DH, Beierwaltes WH. Influence of nitric oxide in the chronic phase of two-kidney, one clip renovascular hypertension. Hypertension. 1998. 31:649–656.
Article6. Soares TJ, Coimbra TM, Martins AR, Pereira AG, Carnio EC, Branco LG, et al. Atrial natriuretic peptide and oxytocin induce natriuresis by release of cGMP. Proc Natl Acad Sci U S A. 1999. 96:278–283.
Article7. Kone BC. Nitric oxide in renal health and disease. Am J Kidney Dis. 1997. 30:311–333.
Article8. Jerkic M, Varagic J, Jovovic D, Radujkovic-Kuburovic G, Nastic-Miric D, Adanja-Grujic G, et al. L-arginine reduces tubular cell injury in acute post-ischaemic renal failure. Nephrol Dial Transplant. 1999. 14:1398–1407.9. Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992. 339:572–575.10. Lee JU. Nitric oxide in the kidney: its physiological role and pathophysiological implications. Electrolyte Blood Press. 2008. 6:27–34.11. John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, et al. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995. 267:679–681.
Article12. Steinhelper ME, Cochrane KL, Field LJ. Hypotension in transgenic mice expressing atrial natriuretic factor fusion genes. Hypertension. 1990. 16:301–307.
Article13. Arai H, Nakao K, Saito Y, Morii N, Sugawara A, Yamada T, et al. Augmented expression of atrial natriuretic polypeptide gene in ventricles of spontaneously hypertensive rats (SHR) and SHR-stroke prone. Circ Res. 1988. 62:926–930.
Article14. Ding J, Thibault G, Gutkowska J, Garcia R, Karabatsos T, Jasmin G, et al. Cardiac and plasma atrial natriuretic factor in experimental congestive heart failure. Endocrinology. 1987. 121:248–257.
Article15. Gutkowska J, Nemer M. Structure, expression, and function of atrial natriuretic factor in extraatrial tissues. Endocr Rev. 1989. 10:519–536.
Article16. Lombardi D, Gordon KL, Polinsky P, Suga S, Schwartz SM, Johnson RJ. Salt-sensitive hypertension develops after short-term exposure to Angiotensin II. Hypertension. 1999. 33:1013–1019.
Article17. Bae EH, Kim IJ, Park JW, Ma SK, Choi KC, Lee JU, et al. Altered regulation of renin-angiotensin, endothelin and natriuretic peptide systems in rat kidney with chronic unilateral ureteral obstruction. Urol Int. 2007. 79:170–176.
Article18. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007. 59:251–287.
Article19. Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev. 2011. 91:1–77.
Article20. Barton M. Therapeutic potential of endothelin receptor antagonists for chronic proteinuric renal disease in humans. Biochim Biophys Acta. 2010. 1802:1203–1213.
Article21. Vaneckova I, Kujal P, Huskova Z, Vanourkova Z, Vernerova Z, Certikova Chabova V, et al. Effects of combined endothelin A receptor and renin-angiotensin system blockade on the course of end-organ damage in 5/6 nephrectomized Ren-2 hypertensive rats. Kidney Blood Press Res. 2012. 35:382–392.
Article22. Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, et al. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994. 78:473–485.
Article23. Nambi P, Pullen M, Wu HL, Aiyar N, Ohlstein EH, Edwards RM. Identification of endothelin receptor subtypes in human renal cortex and medulla using subtype-selective ligands. Endocrinology. 1992. 131:1081–1086.
Article24. Hsu YH, Chen JJ, Chang NC, Chen CH, Liu JC, Chen TH, et al. Role of reactive oxygen species-sensitive extracellular signal-regulated kinase pathway in angiotensin II-induced endothelin-1 gene expression in vascular endothelial cells. J Vasc Res. 2004. 41:64–74.
Article25. Liu Y, Tsuchihashi T, Kagiyama S, Matsumura K, Abe I, Fujishima M. Central and peripheral mechanisms involved in hypertension induced by chronic inhibition of nitric oxide synthase in rats. J Hypertens. 1998. 16:1165–1173.26. Sigmon DH, Newman JM, Beierwaltes WH. Angiotensin II: endothelium-derived nitric oxide interaction in conscious rats. J Am Soc Nephrol. 1994. 4:1675–1682.
Article27. Majid DS, Nishiyama A, Jackson KE, Castillo A. Superoxide scavenging attenuates renal responses to ANG II during nitricoxide synthase inhibition in anesthetized dogs. Am J Physiol Renal Physiol. 2005. 288:F412–F419.28. Ito S, Johnson CS, Carretero OA. Modulation of angiotensin II-induced vasoconstriction by endothelium-derived relaxing factor in the isolated microperfused rabbit afferent arteriole. J Clin Invest. 1991. 87:1656–1663.
Article29. De Nicola L, Blantz RC, Gabbai FB. Nitric oxide and angiotensin II. Glomerular and tubular interaction in the rat. J Clin Invest. 1992. 89:1248–1256.
Article30. McQuillan LP, Leung GK, Marsden PA, Kostyk SK, Kourembanas S. Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanisms. Am J Physiol. 1994. 267(5 Pt 2):H1921–H1927.
Article31. Granger JP, Blaine EH, Stacy DL, La Rock MJ. Effects of long-term increases in plasma ANP on angiotensin II-induced hypertension. Am J Physiol. 1990. 258(5 Pt 2):H1427–H1431.
Article32. Roubert P, Lonchampt MO, Chabrier PE, Plas P, Goulin J, Braquet P. Down-regulation of atrial natriuretic factor receptors and correlation with cGMP stimulation in rat cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1987. 148:61–67.
Article33. Lee JU. Atrial natriuretic peptide attenuates the development of hypertension in 2-kidney, 1-clip goldblatt rats. Korean J Pharmacol. 1989. 23:43–49.34. Goetz KL, Wang BC, Geer PG, Leadley RJ Jr, Reinhardt HW. Atrial stretch increases sodium excretion independently of release of atrial peptides. Am J Physiol. 1986. 250(5 Pt 2):R946–R950.
Article35. Singer DR, Shore AC, Markandu ND, Buckley MG, Sagnella GA, MacGregor GA. Dissociation between plasma atrial natriuretic peptide levels and urinary sodium excretion after intravenous saline infusion in normal man. Clin Sci (Lond). 1987. 73:285–289.
Article36. Bae EH, Ma SK, Lee J, Kim SW. Altered regulation of renal nitric oxide and atrial natriuretic peptide systems in angiotensin II-induced hypertension. Regul Pept. 2011. 170:31–37.
Article37. Wilkins MR, Redondo J, Brown LA. The natriuretic-peptide family. Lancet. 1997. 349:1307–1310.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhanced Atrial Natriuretic Peptide and Nitric Oxide System Following the Treatment with Caffeine in Rats
- Regulatory role of nitric oxide on atrial natriuretic peptide system in normotensive and hypertensive rats
- Blood Pressure Regulation by Vasoactive Peptide Genes: Transgenic And Knockout Animal Models
- Altered Regulation of Renal Nitric Oxide and Atrial Natriuretic Peptide Systems in Lipopolysaccharide-induced Kidney Injury
- Upregulation of Renin-angiotensin, Endothelin and C-type Natriuretic Peptide in Rat Glomerulus with Bilateral Ureteral Obstruction