J Korean Med Assoc.
2011 May;54(5):549-556. 10.5124/jkma.2011.54.5.549.
The burden of illness for meconium aspiration syndrome and cost prediction related to surfactant therapy for meconium aspiration syndrome in Korea
- Affiliations
-
- 1National Evidence-based Healthcare Collaborating Agency, Seoul, Korea. hahns@snu.ac.kr
- 2Department of Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Medical Research Collaborating Center, Seoul National University Hospital, Seoul, Korea.
- 5Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea.
- 6Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea.
- 7Department of Pediatrics, Kyung Hee University College of Medicine, Seoul, Korea.
- KMID: 2190717
- DOI: http://doi.org/10.5124/jkma.2011.54.5.549
Abstract
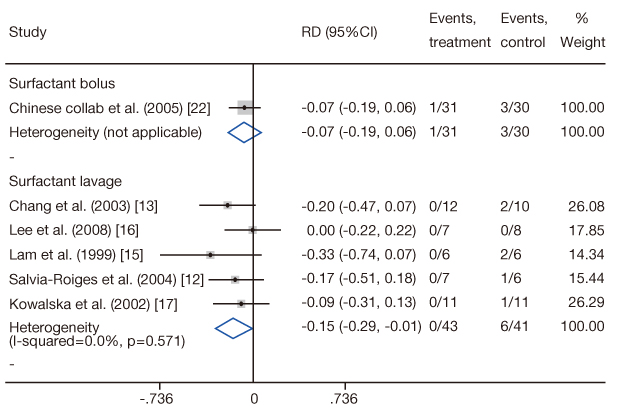
- Meconium aspiration syndrome (MAS) is an important cause of respiratory distress in neonates. Surfactant therapy has been used to improve oxygenation for infants with MAS recently. The object of this study is to estimate the number of candidates for surfactant use in MAS and the cost for surfactant, and to analyze its cost-effectiveness in Korea. Using Korean Health Insurance Review and Assessment Service reimbursement data, the number of neonates with a diagnosis of MAS receiving mechanical ventilation was counted. The annual cost for surfactant use was calculated using the number of patients receiving mechanical ventilation for at least three days who were considered potential candidates for surfactant use. The cost-effectiveness was evaluated using the effectiveness data from a previous meta-analysis. Infants with a diagnosis of MAS receiving mechanical ventilation were 0.92 per 1,000 live births annually. Among them, 55% were potential candidates for surfactant use. The expected annual cost for surfactant was 990 million Korean won and 500 million Korean won and the number needed to treat was 14.3 and 6.7 in surfactant bolus therapy and surfactant lavage therapy, respectively. Sixty-four million won was estimated as the cost to prevent one infant death by surfactant bolus therapy and 15 million won by surfactant lavage therapy. The surfactant therapy for MAS is presently not covered by the Korean national health insurance and its application to MAS is limited because of the high financial burden to the patients' family. This study's results could help healthcare decision makers establish a policy in the future.
MeSH Terms
Figure
Cited by 1 articles
-
Contemporary medical understanding of the 'no-fault accident' during birth: amniotic fluid embolism, pulmonary embolism, meconium aspiration syndrome, and cerebral palsy
Soo-Young Oh, Cheong-Rae Roh
J Korean Med Assoc. 2013;56(9):784-804. doi: 10.5124/jkma.2013.56.9.784.
Reference
-
1. Fleischer A, Anyaegbunam A, Guidetti D, Randolph G, Merkatz IR. A persistent clinical problem: profile of the term infant with significant respiratory complications. Obstet Gynecol. 1992. 79:185–190.2. Dargaville PA, Copnell B. Australian and New Zealand Neonatal Network. The epidemiology of meconium aspiration syndrome: incidence, risk factors, therapies, and outcome. Pediatrics. 2006. 117:1712–1721.
Article3. Davey AM, Becker JD, Davis JM. Meconium aspiration syndrome: physiological and inflammatory changes in a newborn piglet model. Pediatr Pulmonol. 1993. 16:101–108.
Article4. Tran N, Lowe C, Sivieri EM, Shaffer TH. Sequential effects of acute meconium obstruction on pulmonary function. Pediatr Res. 1980. 14:34–38.
Article5. Sun B, Curstedt T, Robertson B. Exogenous surfactant improves ventilation efficiency and alveolar expansion in rats with meconium aspiration. Am J Respir Crit Care Med. 1996. 154(3 Pt 1):764–770.
Article6. Dargaville PA, Mills JF. Surfactant therapy for meconium aspiration syndrome: current status. Drugs. 2005. 65:2569–2591.
Article7. Findlay RD, Taeusch HW, Walther FJ. Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics. 1996. 97:48–52.
Article8. El Shahed AI, Dargaville P, Ohlsson A, Soll RF. Surfactant for meconium aspiration syndrome in full term/near term infants. Cochrane Database Syst Rev. 2007. (3):CD002054.
Article9. Dargaville PA, Copnell B, Mills JF, Haron I, Lee JK, Tingay DG, Rohana J, Mildenhall LF, Jeng MJ, Narayanan A, Battin MR, Kuschel CA, Sadowsky JL, Patel H, Kilburn CJ, Carlin JB, Morley CJ. lessMAS Trial Study Group. Randomized controlled trial of lung lavage with dilute surfactant for meconium aspiration syndrome. J Pediatr. 2011. 158:383–389.e2.
Article10. Wiswell TE, Knight GR, Finer NN, Donn SM, Desai H, Walsh WF, Sekar KC, Bernstein G, Keszler M, Visser VE, Merritt TA, Mannino FL, Mastrioianni L, Marcy B, Revak SD, Tsai H, Cochrane CG. A multicenter, randomized, controlled trial comparing Surfaxin (Lucinactant) lavage with standard care for treatment of meconium aspiration syndrome. Pediatrics. 2002. 109:1081–1087.
Article11. Kattwinkel J. Surfactant lavage for meconium aspiration: a word of caution. Pediatrics. 2002. 109:1167–1168.
Article12. Salvia-Roiges MD, Carbonell-Estrany X, Figueras-Aloy J, Rodriguez-Miguelez JM. Efficacy of three treatment schedules in severe meconium aspiration syndrome. Acta Paediatr. 2004. 93:60–65.
Article13. Chang HY, Hsu CH, Kao HA, Hung HY, Chang JH, Peng CC, Jim WT. Treatment of severe meconium aspiration syndrome with dilute surfactant lavage. J Formos Med Assoc. 2003. 102:326–330.14. Dargaville PA, Mills JF, Copnell B, Loughnan PM, McDougall PN, Morley CJ. Therapeutic lung lavage in meconium aspiration syndrome: a preliminary report. J Paediatr Child Health. 2007. 43:539–545.
Article15. Lam BC, Yeung CY. Surfactant lavage for meconium aspiration syndrome: a pilot study. Pediatrics. 1999. 103(5 Pt 1):1014–1018.
Article16. Lee SM, Kim HM, Jeon JH, Park MS, Park KI, Namgung R, Lee C. Effect of surfactant lavage in severe meconium aspiration syndrome. Korean J Pediatr. 2008. 51:367–371.
Article17. Kowalska K, Szymankiewicz M, Gadzinowski J. An effectiveness of surfactant lung lavage (SLL) in meconium aspiration syndrome (MAS). Przegl Lek. 2002. 59:Suppl 1. 21–24.18. Release of information [Internet]. 2011. cited 2011 Feb 18. Cheongwon: Korea Food and Drug Administration;Available from: http://ezdrug.kfda.go.kr/kfda2.19. HIRA Plus Web [Internet]. cited 2011 Feb 1. Seoul: Health Insurance Review and Assessment Service;Available from: http://www.hira.or.kr/ozjsp/ozmdi.jsp?search_gubun=go_page&page_open=m_bv_exm_std&pgmid=HIRAB030101000000.20. Korea Standard Classification of Disease (KCD) [Internet]. 2010. cited 2010 Sep 1. Daejeon: Korea National Statistical Office;Available from: http://kostat.go.kr/kssc/stclass/StClassAction.do?method=searchDisName&classKind=5&catgrp=kssc&catid1=kssc03&catid2=kssc03b.21. Schlossser RL, Veldman A, Fischer D, Allendorf A, von Loewenich V. Lavage with exogenous surfactant in neonatal meconium aspiration syndrome. Z Geburtshilfe Neonatol. 2002. 206:15–18.22. Chinese Collaborative Study Group for Neonatal Respiratory Diseases. Treatment of severe meconium aspiration syndrome with porcine surfactant: a multicentre, randomized, controlled trial. Acta Paediatr. 2005. 94:896–902.23. Lotze A, Mitchell BR, Bulas DI, Zola EM, Shalwitz RA, Gunkel JH. Survanta in Term Infants Study Group. Multicenter study of surfactant (beractant) use in the treatment of term infants with severe respiratory failure. J Pediatr. 1998. 132:40–47.
Article24. National Evidence-based Healthcare Collaborating Agency. The effect of surfactant therapy for meconium aspiration syndrome. 2011. Seoul: National Evidence-based Healthcare Collaborating Agency.25. Lee SM, Kim HM, Jeon JH, Park MS, Park KI, Namgung R, Lee C. Effect of surfactant lavage in severe meconium aspiration syndrome. Korean J Pediatr. 2008. 51:367–371.
Article26. Park BJ, Sung JH, Park KD, Seo SW, Kim SW. Report of the evaluation for validity of discharged diagnoses in Korean Health Insurance database. 2003. Seoul: Seoul National University.27. Bhutani VK, Chima R, Sivieri EM. Innovative neonatal ventilation and meconium aspiration syndrome. Indian J Pediatr. 2003. 70:421–427.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical observation of meconium aspiration syndrome: prognostic implication of early meconium suctioning
- Surfactant Therapy in Severe Meconium Aspiration Syndrome
- A Study of Meconium Aspiration Syndrome
- A clinical study on meconium-stained babies
- In vitro effect of meconium on the physical surface properties and morphology of exogenous pulmonary surfactant