J Korean Assoc Oral Maxillofac Surg.
2012 Aug;38(4):249-254. 10.5125/jkaoms.2012.38.4.249.
Severe trismus due to bilateral coronoid process hyperplasia in growth hormone therapy patient: a case report
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, School of Dentistry, Pusan National University, Yangsan, Korea. inkchung@pusan.ac.kr
- KMID: 2189633
- DOI: http://doi.org/10.5125/jkaoms.2012.38.4.249
Abstract
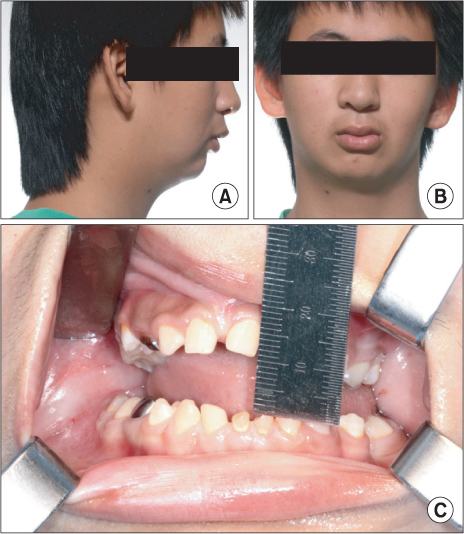
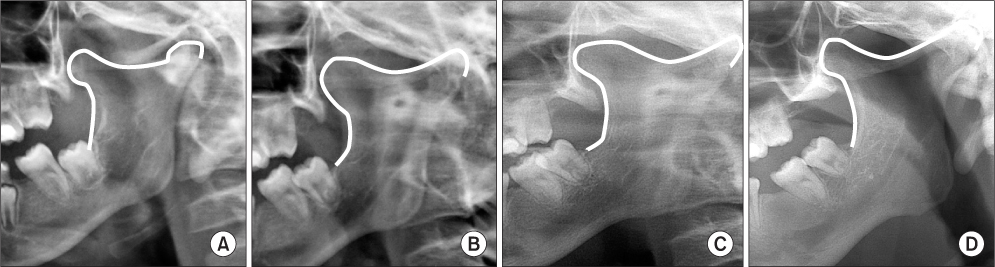
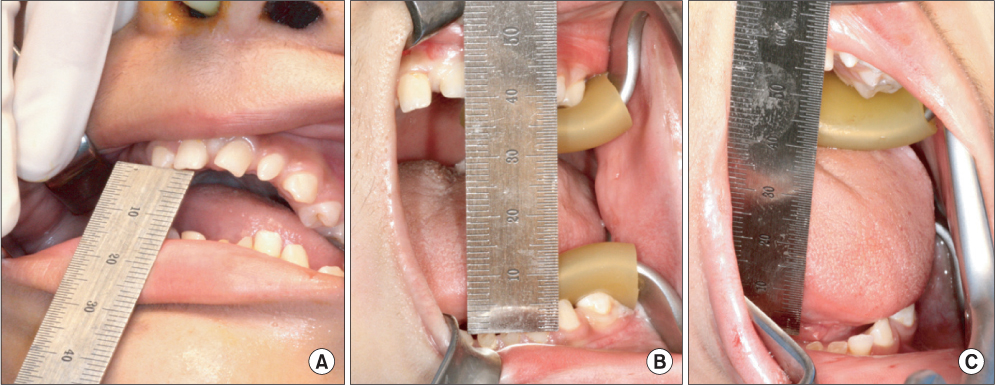
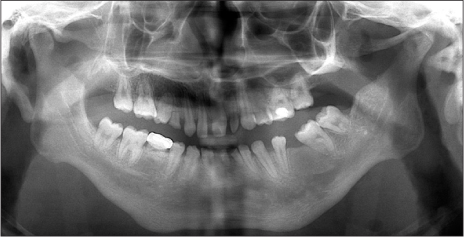
- Bilateral coronoid process hyperplasia is a rare condition characterized by an enlarged mandibular coronoid process. The painless progressive reduction of a mouth opening is caused by coronoid process impingement on the posterior aspect of the zygomatic bone. Hyperplasia of the bilateral coronoid process leads to the restriction of a mandibular opening consequent to the impingement of the enlarged coronoid process on the temporal surface of the zygomatic bone or with the medial surface of the zygomatic arch. The process has been diagnosed as developmental hyperplasia. Otherwise, the development of the coronoid process may be associated with growth hormone. This paper describes a case of trismus caused by coronoid hyperplasia in an idiopathic short-stature patient who received growth hormone therapy by somatropin injections.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A systematic review of treatment and outcomes in patients with mandibular coronoid process hyperplasia
Griet I.L. Parmentier, Margaux Nys, Laurence Verstraete, Constantinus Politis
J Korean Assoc Oral Maxillofac Surg. 2022;48(3):133-148. doi: 10.5125/jkaoms.2022.48.3.133.
Reference
-
1. Isberg A, Isacsson G, Nah KS. Mandibular coronoid process locking: a prospective study of frequency and association with internal derangement of the temporomandibular joint. Oral Surg Oral Med Oral Pathol. 1987. 63:275–279.
Article2. McLoughlin PM, Hopper C, Bowley NB. Hyperplasia of the mandibular coronoid process: an analysis of 31 cases and a review of the literature. J Oral Maxillofac Surg. 1995. 53:250–255.3. Kang HJ, Hwang DS, Kim YD, Shin SH, Kim UK, Kim JR, et al. Clinical study on the etiology, differential diagnosis and treatment of trismus. J Korean Assoc Oral Maxillofac Surg. 2006. 32:544–558.4. Nelson SJ, Nowlin TP, Boeselt B. Consideration of linear and angular values of maximum mandibular opening. Compendium. 1992. 13:362. 364. 366.5. Luyk NH, Steinberg B. Aetiology and diagnosis of clinically evident jaw trismus. Aust Dent J. 1990. 35:523–529.
Article6. Marien M Jr. Trismus: causes, differential diagnosis, and treatment. Gen Dent. 1997. 45:350–355.7. Okeson JP. Orofacial pain: guidelines for assessment, diagnosis, and management. 1996. 3rd ed. Chicago: Quintessence Publishing;45–52.8. Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, et al. 2007 ISS Consensus Workshop participants. 2007 ISS Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008. 93:4210–4217.
Article9. Hintz RL, Attie KM, Baptista J, Roche A. Genentech Collaborative Group. Effect of growth hormone treatment on adult height of children with idiopathic short stature. N Engl J Med. 1999. 340:502–507.
Article10. Spiegel RN, Sather AH, Hayles AB. Cephalometric study of children with various endocrine diseases. Am J Orthod. 1971. 59:362–375.
Article11. Kjellberg H, Beiring M, Albertsson Wikland K. Craniofacial morphology, dental occlusion, tooth eruption, and dental maturity in boys of short stature with or without growth hormone deficiency. Eur J Oral Sci. 2000. 108:359–367.
Article12. van Erum R, Mulier M, Carels C, Verbeke G, de Zegher F. Craniofacial growth in short children born small for gestational age: effect of growth hormone treatment. J Dent Res. 1997. 76:1579–1586.
Article13. Isaksson OG, Lindahl A, Nilsson A, Isgaard J. Mechanism of the stimulatory effect of growth hormone on longitudinal bone growth. Endocr Rev. 1987. 8:426–438.
Article14. Pirinen S, Majurin A, Lenko HL, Koski K. Craniofacial features in patients with deficient and excessive growth hormone. J Craniofac Genet Dev Biol. 1994. 14:144–152.15. Raben MS. Treatment of a pituitary dwarf with human growth hormone. J Clin Endocrinol Metab. 1958. 18:901–903.
Article16. Chung SH, Kim JW, Park YH, Hwang CJ, Lee HK. The effect of growth hormone treatment on craniofacial growth in short stature children. Korean J Orthod. 2010. 40:227–238.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Trismus Due to Bilateral Coronoid Hyperplasia
- Mouth opening limitation caused by coronoid hyperplasia: a report of four cases
- An unusual cause for trismus caused by mandibular coronoid osteoma: a case report
- Coronoid impingement syndrome: literature review and clinical management
- Bilateral elongated mandibular coronoid process in an Anatolian skull