J Korean Orthop Assoc.
2010 Apr;45(2):146-150. 10.4055/jkoa.2010.45.2.146.
Bilateral Femoral Subtrochanteric Insufficiency Fractures after Long-term Bisphosphonate Therapy
- Affiliations
-
- 1Department of Orthopedic Surgery, Chung-Ang University College of Medicine, Seoul, Korea. ksong70@cau.ac.kr
- 2Department of Pathology, Chung-Ang University College of Medicine, Seoul, Korea.
- 3Jincheon Sungmo Hospital, Chungbuk, Korea.
- KMID: 2185583
- DOI: http://doi.org/10.4055/jkoa.2010.45.2.146
Abstract
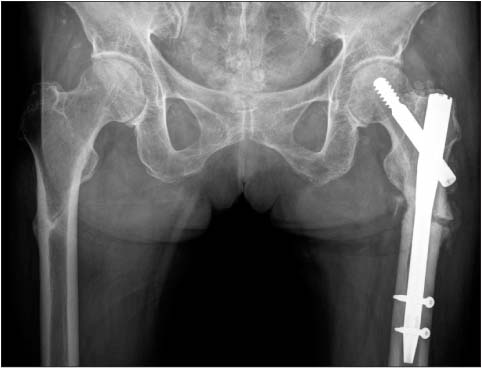
- Bisphosphonates are widely used for the treatment of osteoporosis to prevent fractures. Although their safety and efficacy have been well documented, some recent reports have drawn attention to a possible correlation between long term bisphosphonate therapy and the occurrence of insufficiency fractures owing to prolonged bone turnover suppression. A 71-year-old woman presented to our emergency room with pain and deformity of the left thigh with low energy injury. Radiographs showed a left femur subtrochanteric fracture and a transverse sclerotic fracture line in the right femur subtrochanteric area with cortical thickening, which indicate insufficiency fractures. She had been treated with sodium alendronate 70 mg per week for 6 years and was suffering from prodromal symptoms in both thighs from 3 months ago. After surgery and intraoperative bone biopsy for a left subtrochanteric fracture, her osteoporosis medication was changed with a bone forming agent. We have analyzed the characteristics of insufficiency fractures related to long term bisphosphonate therapy. Physicians should keep in mind the possibility of insufficiency fracture in cases such as ours, especially with prodromal thigh pain.
MeSH Terms
Figure
Cited by 2 articles
-
Treatment and Prognosis of Femoral Insufficiency Fracture Associated with Prolonged Bisphosphonate Use
Ki Chan An, Dae Hyun Park, Guemin Gong, Ju-Young Kim, Sang-Bum Kim, Seung-Yeob Sakong
J Korean Fract Soc. 2014;27(1):10-16. doi: 10.12671/jkfs.2014.27.1.10.Bilateral Femoral Neck Insufficiency Fractures after Use of a Long-term Anti-resorptive Drug Therapy for Osteoporosis: A Case Report
Dong-Ki Ahn, Jin-Hak Kim, Jae-Il Lee, Jin-Woo Kim
Hip Pelvis. 2015;27(2):115-119. doi: 10.5371/hp.2015.27.2.115.
Reference
-
1. Miller PD, Watts NB, Licata AA, et al. Cyclical etidronate in the treatment of postmenopausal osteoporosis: efficacy and safety after seven years of treatment. Am J Med. 1997. 103:468–476.2. Bone HG, Hosking D, Devogelaer JP, et al. Ten year's experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004. 350:1189–1199.
Article3. Silverman SL, Watts NB, Delmas PD, Lange JL, Lindsay R. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007. 18:25–34.
Article4. Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998. 13:581–589.
Article5. Currey JD. Effects of differences in mineralization on the mechanical properties of bone. Philos Trans R Soc Lond B Biol Sci. 1984. 304:509–518.6. Wasserman N, Yerramshetty J, Akkus O. Microcracks colocalize within highly mineralized regions of cortical bone tissue. Eur J Morphol. 2005. 42:43–51.
Article7. Kwek EB, Goh SK, Koh JS, Png MA, Howe TS. An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury. 2008. 39:224–231.
Article8. Black DM, Cummings SR, Karpf DB, et al. Fracture Intervention Trial Research Group. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996. 348:1535–1541.
Article9. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005. 90:1294–1301.
Article10. Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006. 296:2927–2938.11. Ott SM. Fractures after long-term alendronate therapy. J Clin Endocrinol Metab. 2001. 86:1835–1836.
Article12. Schneider JP. Should bisphosphonates be continued indefinitely? An unusual fracture in a healthy woman on long-term alendronate. Geriatrics. 2006. 61:31–33.13. Iwamoto J, Takeda T. Insufficiency fracture of the femoral neck during osteoporosis treatment: a case report. J Orthop Sci. 2002. 7:707–712.
Article14. Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008. 22:346–350.
Article15. Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest. 1997. 100:1475–1480.
Article16. Armamento-Villareal R, Napoli N, Panwar V, Novack D. Suppressed bone turnover during alendronate therapy for high-turnover osteoporosis. N Engl J Med. 2006. 355:2048–2050.
Article17. Yang KH, Sim DS. Clinical consideration on insufficiency fracture of femur. Korean Journal of Bone Metabolism. 2009. 16:37–41.18. Burr DB. Remodeling and the repair of fatigue damage. Calcif Tissue Int. 1993. 53:Suppl. 75–81.
Article19. Pleiner-Duxneuner J, Zwettler E, Paschalis E, Roschger P, Nell-Duxneuner V, Klaushofer K. Treatment of osteoporosis with parathyroid hormone and teriparatide. Calcif Tissue Int. 2009. 84:159–170.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidemiology and Clinical Features of Atypical Femoral Fractures
- Is It a Simple Stress Fracture or Bisphosphonate-related Atypical Fracture?
- Sequential Bilateral Insufficiency Fractures of the Femur Neck in Patients Treated with Prolonged Bisphosphonate Therapy: A Case Report
- A Case Report of Long-Term Bisphosphonate Therapy and Atypical Stress Fracture of Bilateral Femur
- Insufficiency Fracture of Simultaneously Bilateral Femur Neck in Patient Treated with Long-Term Bisphosphonate Treatment - A Case Report -