J Korean Orthop Assoc.
2012 Dec;47(6):439-444. 10.4055/jkoa.2012.47.6.439.
Total Hip Arthroplasty Using AML(R) Prosthesis: Minimum 20-Year Follow-Up of the Patients
- Affiliations
-
- 1Department of Orthopaedic Surgery, Kwangju Christian Hospital, Gwangju, Korea. paedic@chol.com
- KMID: 2185307
- DOI: http://doi.org/10.4055/jkoa.2012.47.6.439
Abstract
- PURPOSE
We reviewed the radiological outcomes and survival rate of the total hip arthroplasty (THA) with AML(R) (Anatomic Medullary Locking, DePuy, Warsaw, IN, USA) hip prosthesis on long-term follow-up.
MATERIALS AND METHODS
From May 1988 to December 1990, 93 hip arthroplasties were performed on 77 patients in our hospital. In this study, 30 patients, of whom 41 hips underwent the procedure, were alive and able to be included. Follow-up was average of 21.4 years. The mean patient age was 45 years (35-60 years) at the time of operation. Of the hip procedures included in our study, the reasons for THA were osteonecrosis of the femoral head in 25 hips, rheumatoid arthritis in 3 and acetabular dysplasia in 2. We analyzed the wear rate of the polyethylene, osteolysis of the femur and acetabulum and stress shielding of the femur on the follow-up radiographs. In addition, we investigated the survival rate of the prosthesis and causes of revision in the last follow-up.
RESULTS
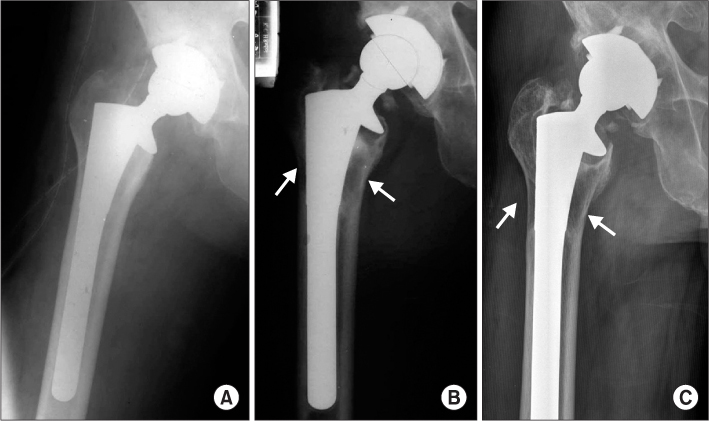
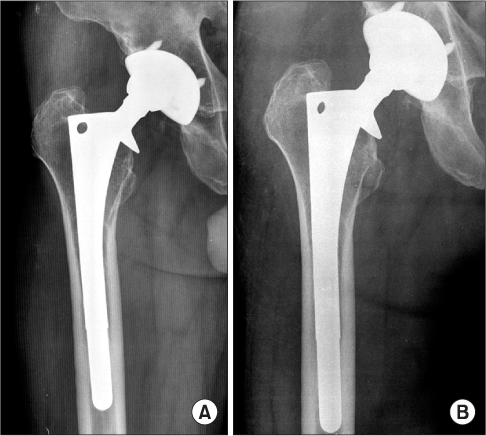
The polyethylene wear rate of the surviving acetabular cup was 0.15 mm/yr. Acetabular osteolysis was detected in 33 hips and was mostly in zone 2 and 3. Femoral osteolysis was showed in 32 hips in zone 1 and 7. Stress shielding over grade 3 was found in 5 of 21 femoral stems in over 13.5 mm in diameter. The grade of stress shielding did not progress with follow-up. Of the 33 hips, 26 (63.4%) cups were revised for polyethylene wear and osteolysis. There were 6 (21%) femoral stems revised for osteolysis.
CONCLUSION
The cause of a high revision rate of the prosthesis was polyethylene wear and osteolysis. We predict that THA using AML(R) prosthesis with wear-resistant bearing surfaces could increase the survival rate on long-term follow-up over 20 years.
Keyword
MeSH Terms
Figure
Reference
-
1. DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976. (121):20–32.
Article2. Goldring SR, Schiller AL, Roelke M, Rourke CM, ONeil DA, Harris WH. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg Am. 1983. 65:575–584.
Article3. Cook SD, Barrack RL, Thomas KA, Haddad RJ Jr. Quantitative analysis of tissue growth into human porous total hip components. J Arthroplasty. 1988. 3:249–262.
Article4. Judet R, Siguier M, Brumpt B, Judet T. A noncemented total hip prosthesis. Clin Orthop Relat Res. 1978. (137):76–84.
Article5. Callaghan JJ, Templeton JE, Liu SS, Warth LC, Chung YY. Improved results using extensively coated THA stems at minimum 5-year followup. Clin Orthop Relat Res. 2006. 453:91–96.
Article6. Kim I, Kim JM, Kim YS, Kim SS, Eun SP. Clinical study of AML total hip replacement arthroplasty. J Korean Orthop Assoc. 1991. 26:886–891.
Article7. Callaghan JJ, Dysart SH, Savory CG. The uncemented porous-coated anatomic total hip prosthesis. Two-year results of a prospective consecutive series. J Bone Joint Surg Am. 1988. 70:337–346.
Article8. Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987. 69:45–55.
Article9. Engh CA, Massin P. Cementless total hip arthroplasty using the anatomic medullary locking stem. Results using a survivorship analysis. Clin Orthop Relat Res. 1989. (249):141–158.
Article10. Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001. (393):137–146.
Article11. Kang JS, Moon KH, Park SR, Choi SW. Long-term results of total hip arthroplasty with an extensively porous coated stem in patients younger than 45 years old. Yonsei Med J. 2010. 51:100–103.
Article12. Belmont PJ Jr, Powers CC, Beykirch SE, Hopper RH Jr, Engh CA Jr, Engh CA. Results of the anatomic medullary locking total hip arthroplasty at a minimum of twenty years. A concise follow-up of previous reports. J Bone Joint Surg Am. 2008. 90:1524–1530.
Article13. Gruen TA, McNeice GM, Amstutz HC. "Modes of failure" of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979. (141):17–27.
Article14. Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990. 72:518–528.15. Kim YH, Kim VE. Uncemented porous-coated anatomic total hip replacement. Results at six years in a consecutive series. J Bone Joint Surg Br. 1993. 75:6–13.16. Kim YH, Kim JS, Cho SH. Primary total hip arthroplasty with the AML total hip prosthesis. Clin Orthop Relat Res. 1999. (360):147–158.
Article17. Haddad RJ, Cook SD, Brinker MR. A comparison of three varieties of noncemented porous-coated hip replacement. J Bone Joint Surg Br. 1990. 72:2–8.
Article18. Hwang SK, Park JS. Cementless total hip arthroplasty with AML, PCA and HGP prostheses. Int Orthop. 1995. 19:77–83.
Article19. Keaveny TM, Bartel DL. Effects of porous coating, with and without collar support, on early relative motion for a cementless hip prosthesis. J Biomech. 1993. 26:1355–1368.
Article20. Kim YH, Oh JH, Oh SH. Cementless total hip arthroplasty in patients with osteonecrosis of the femoral head. Clin Orthop Relat Res. 1995. (320):73–84.21. Kim YH, Kim JS, Cho SH. Primary total hip arthroplasty with a cementless porous-coated anatomic total hip prosthesis: 10- to 12-year results of prospective and consecutive series. J Arthroplasty. 1999. 14:538–548.22. Ikema M, Oyama M, Kita A, Funayama K. Poor results of the cementless total hip arthroplasty with a nonporous coated acetabular component: AcSys Shearer Cup. J Orthop Sci. 2007. 12:466–470.
Article23. Moyer JA, Metz CM, Callaghan JJ, Hennessy DW, Liu SS. Durability of second-generation extensively porous-coated stems in patients age 50 and younger. Clin Orthop Relat Res. 2010. 468:448–453.
Article24. Nourbash PS, Paprosky WG. Cementless femoral design concerns. Rationale for extensive porous coating. Clin Orthop Relat Res. 1998. (355):189–199.
Article25. Stöhr KK, Phillips C, Healy JC, Al-Yassiri G, Gibbons CE. Medium-to-long term DEXA analysis of an uncemented (AML) femoral component. Hip Int. 2008. 18:195–199.
Article26. Hartzband MA, Glassman AH, Goldberg VM, Jordan LR, Crowninshield RD, Fricka KB, et al. Survivorship of a low-stiffness extensively porous-coated femoral stem at 10 years. Clin Orthop Relat Res. 2010. 468:433–440.
Article27. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001. (393):128–136.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Clinical and Radiologic Results of Total Hip Arthroplasty with an AML Hip Prosthesis: Minimum 7 Years' Follow-up Study
- Cementless Total Hip Arthroplasty with Use of the COREN Hip System
- Result of a Minimum Five-Year Follow-Up of Hip Arthroplasty Using the Bencox(R) Hip Stem
- Total Hip replacement with AML Prosthesis for Avascular Necrosis of the Femoral Head
- Cementless Total Hip Arthroplasty Using the ABG I Hydroxyapatite-coated Prosthesis: Minimum 10 Year Follow-up