J Breast Cancer.
2015 Dec;18(4):386-393. 10.4048/jbc.2015.18.4.386.
Independent Prognostic Factors for Overall Survival after Salvage Operation for Ipsilateral Breast Tumor Recurrence Following Breast-Conserving Surgery
- Affiliations
-
- 1Department of Surgery, Samsung Biomedical Research Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. seokjin.nam@samsung.com
- KMID: 2176288
- DOI: http://doi.org/10.4048/jbc.2015.18.4.386
Abstract
- PURPOSE
Few studies address independent prognostic factors after ipsilateral breast tumor recurrence (IBTR) following breast-conserving surgery (BCS). Locoregional recurrence is associated with distant metastases and increased mortality rates. Therefore anticipating prognoses after IBTR and evaluating risk factors for overall survival following a second salvage operation are important. We evaluated independent prognostic factors affecting overall survival after a second operation for IBTR.
METHODS
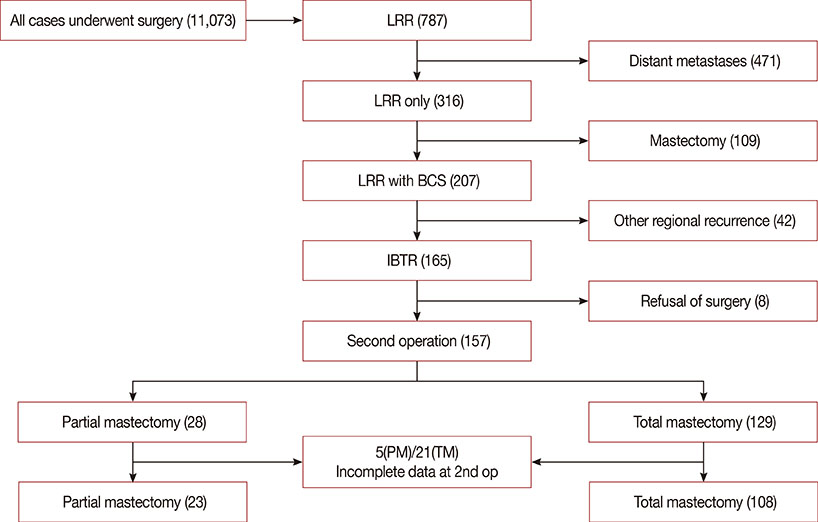
We retrospectively identified 11,073 patients who underwent breast cancer surgery between November 1995 and December 2011. Locoregional recurrence occurred in 787 patients. Among them, IBTR developed in 165 patients selected for analysis. Excluding eight patients who refused further treatment, we analyzed 157 patients who underwent a second operation (partial mastectomy, 28 [17.8%]; total mastectomy, 129 [82.2%]) for IBTR. Excluding 26 patients with incomplete data, we evaluated the clinicopathol-ogical features influencing overall survival at the first and the second operation in the 131 patients who underwent a second operation.
RESULTS
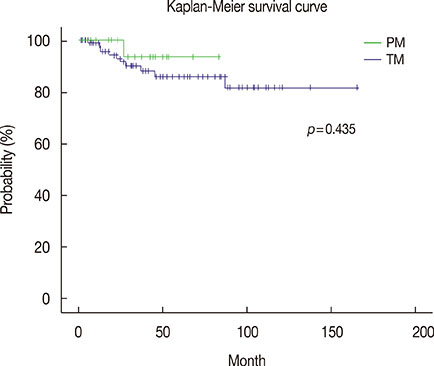
The median age of patients at the first operation was 43.6 years (range, 27-69 years). The median duration from the first to the second operation was 45.0 months (range, 2.5-164.6 months). The 5-year overall survival rate after IBTR was 87.1%. In the multivariable analyses, duration from the first to the second operation, histopathology, lymph node status, and adjuvant chemotherapy, radiotherapy, and endocrine therapy at the first operation were independent prognostic factors for overall survival. Positive estrogen receptor status and endocrine therapy at the second operation were also associated with increased overall survival following salvage operations for IBTR.
CONCLUSION
The time interval to IBTR following BCS is related to overall survival after salvage operation for IBTR and it is important to undergo optimal adjuvant treatments according to risk factors after the first operation because those risk factors affect overall survival for IBTR following BCS.
MeSH Terms
Figure
Reference
-
1. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347:1233–1241.
Article2. Arriagada R, Lê MG, Rochard F, Contesso G. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol. 1996; 14:1558–1564.
Article3. Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995; 333:1456–1461.
Article4. Lichter AS, Lippman ME, Danforth DN Jr, d'Angelo T, Steinberg SM, deMoss E, et al. Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: a randomized trial at the National Cancer Institute. J Clin Oncol. 1992; 10:976–983.
Article5. van der Sangen MJ, Poortmans PM, Scheepers SW, Lemaire BM, van Berlo CL, Tjan-Heijnen VC, et al. Prognosis following local recurrence after breast conserving treatment in young women with early breast cancer. Eur J Surg Oncol. 2013; 39:892–898.
Article6. Tanis E, van de Velde CJ, Bartelink H, van de Vijver MJ, Putter H, van der Hage JA. Locoregional recurrence after breast-conserving therapy remains an independent prognostic factor even after an event free interval of 10 years in early stage breast cancer. Eur J Cancer. 2012; 48:1751–1756.
Article7. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002; 347:1227–1232.
Article8. van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000; 92:1143–1150.
Article9. Anderson SJ, Wapnir I, Dignam JJ, Fisher B, Mamounas EP, Jeong JH, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009; 27:2466–2473.
Article10. Wapnir IL, Anderson SJ, Mamounas EP, Geyer CE Jr, Jeong JH, Tan-Chiu E, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006; 24:2028–2037.
Article11. Whelan T, Clark R, Roberts R, Levine M, Foster G. Ipsilateral breast tumor recurrence postlumpectomy is predictive of subsequent mortality: results from a randomized trial. Investigators of the Ontario Clinical Oncology Group. Int J Radiat Oncol Biol Phys. 1994; 30:11–16.
Article12. Alpert TE, Kuerer HM, Arthur DW, Lannin DR, Haffty BG. Ipsilateral breast tumor recurrence after breast conservation therapy: outcomes of salvage mastectomy vs. salvage breast-conserving surgery and prognostic factors for salvage breast preservation. Int J Radiat Oncol Biol Phys. 2005; 63:845–851.
Article13. Salvadori B, Marubini E, Miceli R, Conti AR, Cusumano F, Andreola S, et al. Reoperation for locally recurrent breast cancer in patients previously treated with conservative surgery. Br J Surg. 1999; 86:84–87.
Article14. Dalberg K, Mattsson A, Sandelin K, Rutqvist LE. Outcome of treatment for ipsilateral breast tumor recurrence in early-stage breast cancer. Breast Cancer Res Treat. 1998; 49:69–78.
Article15. Abner AL, Recht A, Eberlein T, Come S, Shulman L, Hayes D, et al. Prognosis following salvage mastectomy for recurrence in the breast after conservative surgery and radiation therapy for early-stage breast cancer. J Clin Oncol. 1993; 11:44–48.
Article16. van der Sangen MJ, van de Poll-Franse LV, Roumen RM, Rutten HJ, Coebergh JW, Vreugdenhil G, et al. The prognosis of patients with local recurrence more than five years after breast conservation therapy for invasive breast carcinoma. Eur J Surg Oncol. 2006; 32:34–38.
Article17. Connor CS, Touijer AK, Krishnan L, Mayo MS. Local recurrence following breast conservation therapy in African-American women with invasive breast cancer. Am J Surg. 2000; 179:22–26.
Article18. Fowble B, Solin LJ, Schultz DJ, Rubenstein J, Goodman RL. Breast recurrence following conservative surgery and radiation: patterns of failure, prognosis, and pathologic findings from mastectomy specimens with implications for treatment. Int J Radiat Oncol Biol Phys. 1990; 19:833–842.
Article19. Voogd AC, van Tienhoven G, Peterse HL, Crommelin MA, Rutgers EJ, van de Velde CJ, et al. Local recurrence after breast conservation therapy for early stage breast carcinoma: detection, treatment, and outcome in 266 patients. Dutch Study Group on Local Recurrence after Breast Conservation (BORST). Cancer. 1999; 85:437–446.
Article20. Gage I, Schnitt SJ, Recht A, Abner A, Come S, Shulman LN, et al. Skin recurrences after breast-conserving therapy for early-stage breast cancer. J Clin Oncol. 1998; 16:480–486.
Article21. Haffty BG, Reiss M, Beinfield M, Fischer D, Ward B, McKhann C. Ipsilateral breast tumor recurrence as a predictor of distant disease: implications for systemic therapy at the time of local relapse. J Clin Oncol. 1996; 14:52–57.
Article22. Veronesi U, Marubini E, Del Vecchio M, Manzari A, Andreola S, Greco M, et al. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst. 1995; 87:19–27.
Article23. Rouzier R, Extra JM, Carton M, Falcou MC, Vincent-Salomon A, Fourquet A, et al. Primary chemotherapy for operable breast cancer: incidence and prognostic significance of ipsilateral breast tumor recurrence after breast-conserving surgery. J Clin Oncol. 2001; 19:3828–3835.
Article24. Komoike Y, Akiyama F, Iino Y, Ikeda T, Akashi-Tanaka S, Ohsumi S, et al. Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer: risk factors and impact on distant metastases. Cancer. 2006; 106:35–41.
Article25. Halverson KJ, Perez CA, Kuske RR, Garcia DM, Simpson JR, Fineberg B. Survival following locoregional recurrence of breast cancer: univariate and multivariate analysis. Int J Radiat Oncol Biol Phys. 1992; 23:285–291.
Article26. Kurtz JM, Jacquemier J, Amalric R, Brandone H, Ayme Y, Hans D, et al. Is breast conservation after local recurrence feasible? Eur J Cancer. 1991; 27:240–244.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Disease Free Survival and Prognostic Factors for Patients with Breast Conserving Surgery
- Surgical Options for Ipsilateral Breast Tumor Recurrence: Mastectomy Versus Repeat Breast-Conserving Surgery
- Multiple Margin Positivity of Frozen Section Is an Independent Risk Factor for Local Recurrence in Breast-Conserving Surgery
- Recurrent and Second Breast Cancer Detected on Follow-Up Mammography and Breast Ultrasound after Breast-Conserving Surgery: Imaging Findings and Clinicopathologic Factors
- Prognostic Value of the Preoperative CEA, CA15-3 and TPS Serum Levels in Patients with Breast Cancer