Investig Magn Reson Imaging.
2015 Jun;19(2):76-87. 10.13104/imri.2015.19.2.76.
Effect of Manganese Content on the Magnetic Susceptibility of Ferrous-Manganese Alloys: Correlation between Microstructure on X-Ray Diffraction and Size of the Low-Intensity Area on MRI
- Affiliations
-
- 1Department of Radiology, Catholic University of Daegu, School of Medicine, Daegu, Korea. ysw10adest@cu.ac.kr
- 2Department of Materials Science and Metallurgical Engineering, Kyungpook National University, Daegu, Korea.
- 3Department of Pathology, Catholic University of Daegu, School of Medicine, Daegu, Korea.
- 4Department of Radiology, Kyungpook National University, School of Medicine, Daegu, Korea.
- 5Korea Institute of Industrial Technology Dongnam Technology Application Division, Pusan, Korea.
- KMID: 2175587
- DOI: http://doi.org/10.13104/imri.2015.19.2.76
Abstract
- PURPOSE
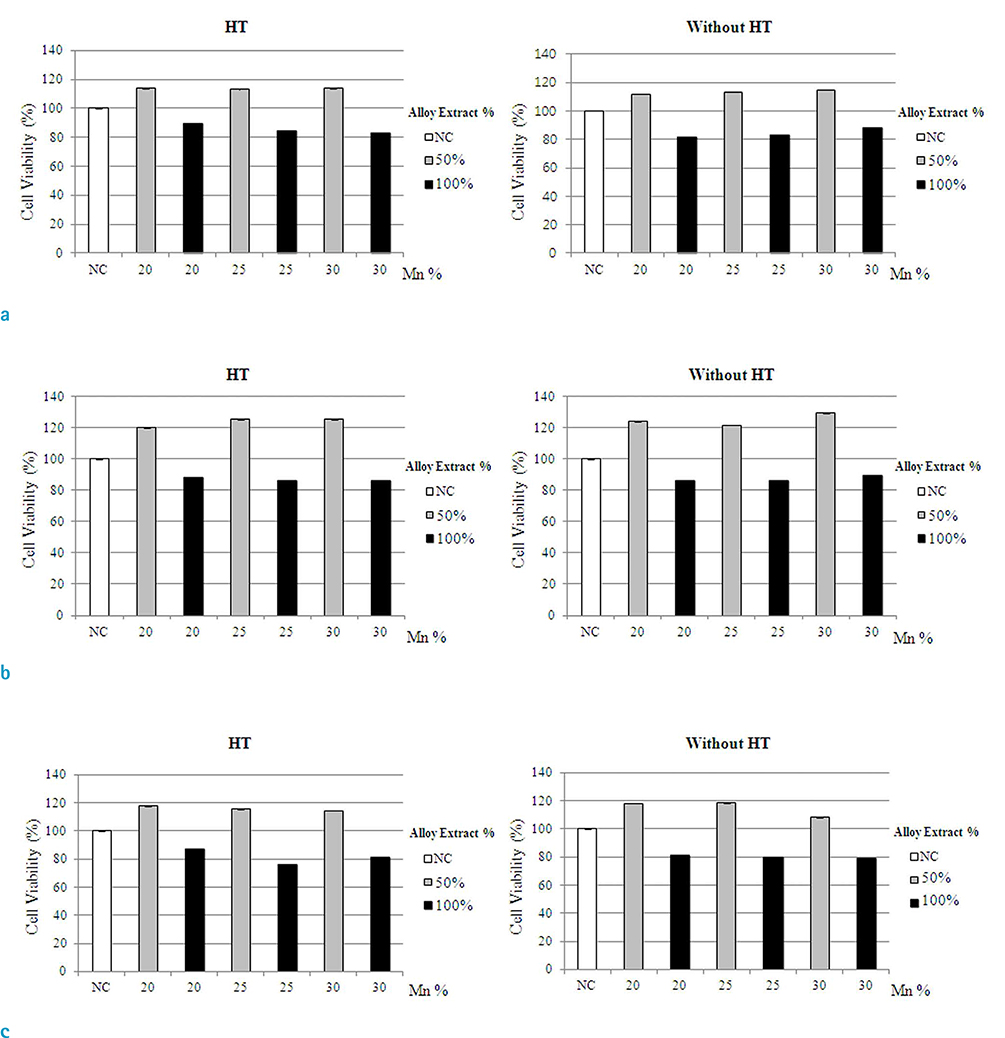
There is an ongoing search for a stent material that produces a reduced susceptibility artifact. This study evaluated the effect of manganese (Mn) content on the MRI susceptibility artifact of ferrous-manganese (Fe-Mn) alloys, and investigated the correlation between MRI findings and measurements of Fe-Mn microstructure on X-ray diffraction (XRD).
MATERIALS AND METHODS
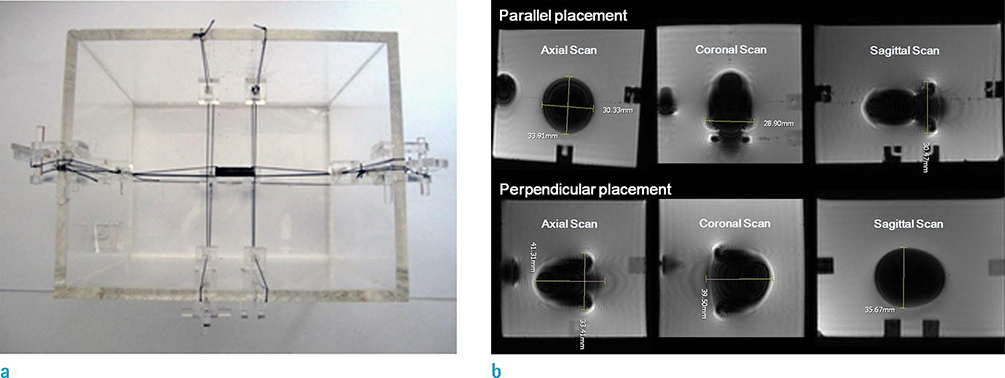
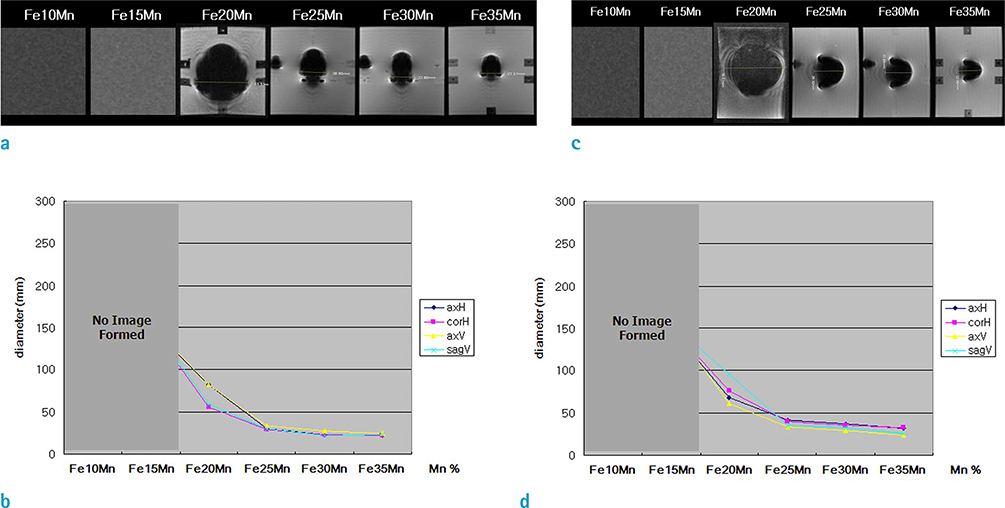
Fe-Mn binary alloys were prepared with Mn contents varying from 10% to 35% by weight (i.e., 10%, 15%, 20%, 25%, 30%, and 35%; designated as Fe-10Mn, Fe-15Mn, Fe-20Mn, Fe-25Mn, Fe-30Mn, and Fe-35Mn, respectively), and their microstructure was evaluated using XRD. Three-dimensional spoiled gradient echo sequences of cylindrical specimens were obtained in parallel and perpendicular to the static magnetic field (B0). In addition, T1-weighted spin echo, T2-weighted fast spin echo, and T2*-weighted gradient echo images were obtained. The size of the low-intensity area on MRI was measured for each of the Fe-Mn binary alloys prepared.
RESULTS
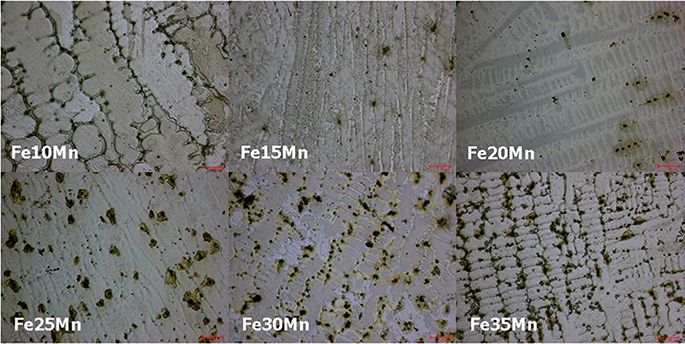
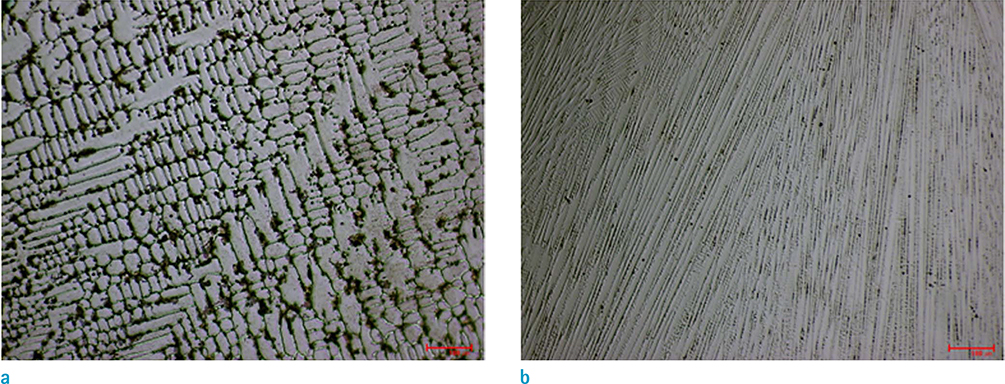
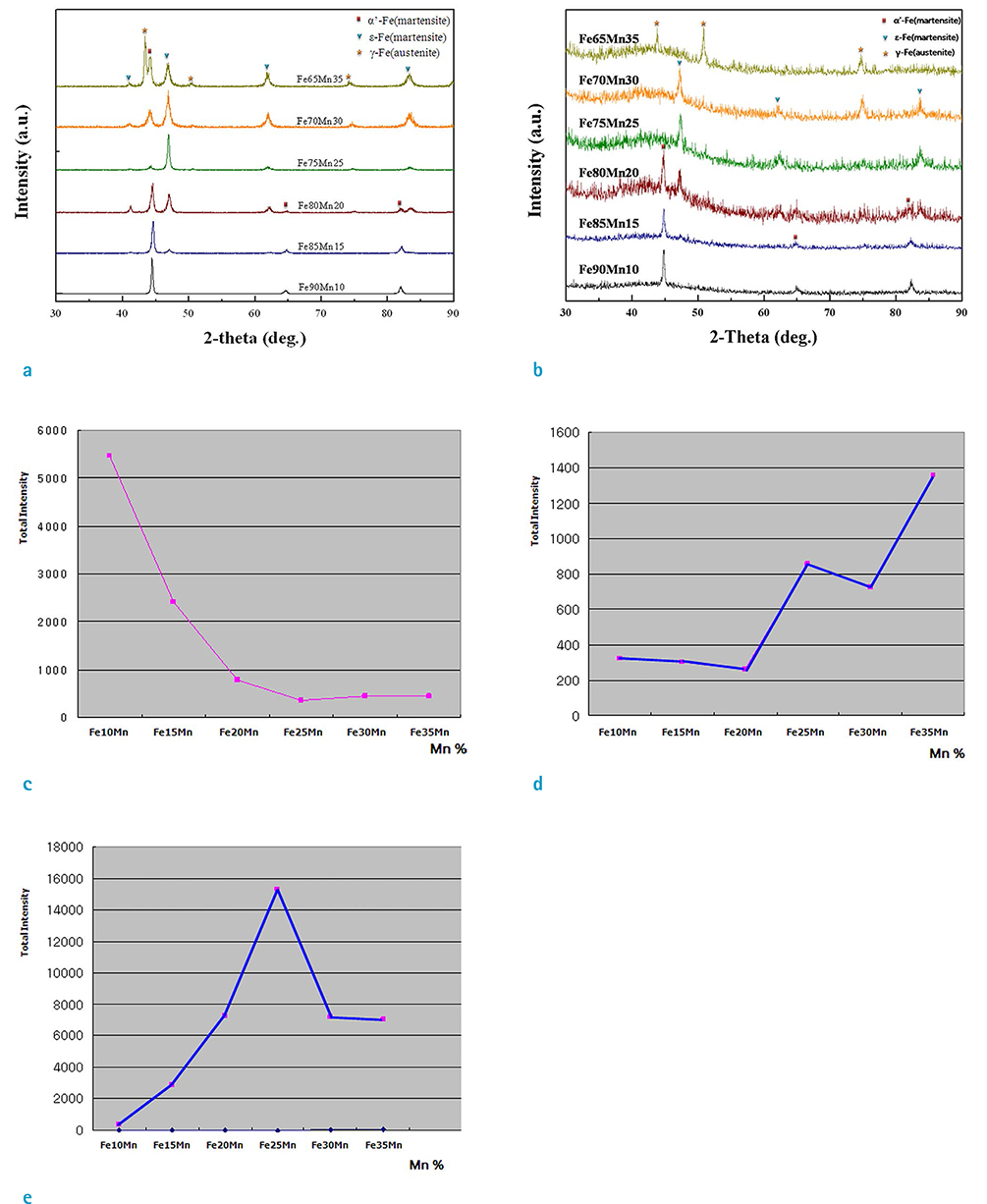
Three phases of alpha'-martensite, gamma-austenite, and epsilon-martensite were seen on XRD, and their composition changed from alpha'-martensite to gamma-austenite and/or epsilon-martensite, with increasing Mn content. The Fe-10Mn and Fe-15Mn specimens comprised alpha'-martensite, the Fe-20Mn and Fe-25Mn specimens comprised gamma+epsilon phases, and the Fe-30Mn and Fe-35Mn specimens exhibited a single gamma phase. The size of the low-intensity areas of Fe-Mn on MRI decreased relative to its microstructure on XRD with increasing Mn content.
CONCLUSION
Based on these findings, proper conditioning of the Mn content in Fe-Mn alloys will improve its visibility on MR angiography, and a Mn content of more than 25% is recommended to reduce the magnetic susceptibility artifacts on MRI. A reduced artifact of Fe-Mn alloys on MRI is closely related to the paramagnetic constitution of gamma-austenite and/or epsilon-martensite.
Keyword
MeSH Terms
Figure
Reference
-
1. Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987; 316:701–706.2. Schomig A, Kastrati A, Mudra H, et al. Four-year experience with Palmaz-Schatz stenting in coronary angioplasty complicated by dissection with threatened or present vessel closure. Circulation. 1994; 90:2716–2724.3. Hoffmann R, Mintz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation. 1996; 94:1247–1254.4. Heublein B, Rohde R, Kaese V, Niemeyer M, Hartung W, Haverich A. Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology? Heart. 2003; 89:651–656.5. Waksman R, Pakala R, Kuchulakanti PK, et al. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries. Catheter Cardiovasc Interv. 2006; 68:607–617. discussion 618-6196. Waksman R. Update on bioabsorbable stents: from bench to clinical. J Interv Cardiol. 2006; 19:414–421.7. Erne P, Schier M, Resink TJ. The road to bioabsorbable stents: reaching clinical reality? Cardiovasc Intervent Radiol. 2006; 29:11–16.8. Peuster M, Wohlsein P, Brugmann M, et al. A novel approach to temporary stenting: degradable cardiovascular stents produced from corrodible metal-results 6-18 months after implantation into New Zealand white rabbits. Heart. 2001; 86:563–569.9. Peuster M, Hesse C, Schloo T, Fink C, Beerbaum P, von Schnakenburg C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials. 2006; 27:4955–4962.10. Hermawan H, Dube D, Mantovani D. Degradable metallic biomaterials: design and development of Fe-Mn alloys for stents. J Biomed Mater Res A. 2010; 93:1–11.11. Schinhammer M, Hanzi AC, Loffler JF, Uggowitzer PJ. Design strategy for biodegradable Fe-based alloys for medical applications. Acta Biomater. 2010; 6:1705–1713.12. Liu B, Zheng YF. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomater. 2011; 7:1407–1420.13. Buecker A, Spuentrup E, Ruebben A, Gunther RW. Artifact-free in-stent lumen visualization by standard magnetic resonance angiography using a new metallic magnetic resonance imaging stent. Circulation. 2002; 105:1772–1775.14. Trost DW, Zhang HL, Prince MR, et al. Three-dimensional MR angiography in imaging platinum alloy stents. J Magn Reson Imaging. 2004; 20:975–980.15. O'Brien BJ, Stinson JS, Boismier DA, Carroll WM. Characterization of an NbTaWZr alloy designed for magnetic resonance angiography compatible stents. Biomaterials. 2008; 29:4540–4545.16. O'Brien B, Stinson J, Carroll W. Development of a new niobium-based alloy for vascular stent applications. J Mech Behav Biomed Mater. 2008; 1:303–312.17. American Society for Testing and Materials (ASTM) standard F2119-07. Standard test method for evaluation of MR image artifacts from passive implants. 2013. Accessed June 19, 2015. http://www.astm.org/Standards/F2119.htm.18. Port JD, Pomper MG. Quantification and minimization of magnetic susceptibility artifacts on GRE images. J Comput Assist Tomogr. 2000; 24:958–964.19. Wang Y, Truong TN, Yen C, et al. Quantitative evaluation of susceptibility and shielding effects of nitinol, platinum, cobalt-alloy, and stainless steel stents. Magn Reson Med. 2003; 49:972–976.20. Coecke S, Balls M, Bowe G, et al. Guidance on good cell culture practice. a report of the second ECVAM task force on good cell culture practice. Altern Lab Anim. 2005; 33:261–287.21. Gu X, Zheng Y, Cheng Y, Zhong S, Xi T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials. 2009; 30:484–498.22. Choi JW, Roh HG, Moon WJ, et al. Time-resolved 3D contrast-enhanced MRA on 3.0T: a non-invasive follow-up technique after stent-assisted coil embolization of the intracranial aneurysm. Korean J Radiol. 2011; 12:662–670.23. Takayama K, Taoka T, Nakagawa H, et al. Usefulness of contrast-enhanced magnetic resonance angiography for follow-up of coil embolization with the enterprise stent for cerebral aneurysms. J Comput Assist Tomogr. 2011; 35:568–572.24. Seok JH, Choi HS, Jung SL, et al. Artificial luminal narrowing on contrast-enhanced magnetic resonance angiograms on an occasion of stent-assisted coiling of intracranial aneurysm: in vitro comparison using two different stents with variable imaging parameters. Korean J Radiol. 2012; 13:550–556.25. Crossgrove J, Zheng W. Manganese toxicity upon overexposure. NMR Biomed. 2004; 17:544–553.26. Reaney SH, Bench G, Smith DR. Brain accumulation and toxicity of Mn(II) and Mn(III) exposures. Toxicol Sci. 2006; 93:114–124.27. Chang Y, Jin SU, Kim Y, et al. Decreased brain volumes in manganese-exposed welders. Neurotoxicology. 2013; 37:182–189.28. Chen JY, Tsao GC, Zhao Q, Zheng W. Differential cytotoxicity of Mn(II) and Mn(III): special reference to mitochondrial [Fe-S] containing enzymes. Toxicol Appl Pharmacol. 2001; 175:160–168.29. Hermawan H, Purnama A, Dube D, Couet J, Mantovani D. Fe-Mn alloys for metallic biodegradable stents: degradation and cell viability studies. Acta Biomater. 2010; 6:1852–1860.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on the Manganese Exposure and Health Hazard among Manganese Manufacturing Woman Workers
- Significance of Brain Magnetic Resonance Imaging (MRI) in the Assessment of Occupational Manganese Exposure
- Study on Clinical Significance of High Signal Intensity by Brain Magnetic Resonance Imaging in Mild Steel/Arc Welders (Clinical Significance of High Signal Intensity by Brain MRI in Welders)
- Relationship of Biological Indices of Manganese with Pallidal Index on MRI in Liver Cirrhotics
- The Change of Brain MRI and Pathology According to the Administered Dose of Manganese in Rats