J Gynecol Oncol.
2011 Dec;22(4):275-287. 10.3802/jgo.2011.22.4.275.
Diffusion-weighted MR imaging in gynecologic cancers
- Affiliations
-
- 1Department of Radiology, Takagi Hospital, Okawa, Japan. s.motoshima@gmail.com
- 2Department of Radiology, Saga University Faculty of Medicine, Saga, Japan.
- 3Department of Radiology, Saga Social Insurance Hospital, Saga, Japan.
- 4Department of Obstetrics and Gynecology, Kurume University School of Medicine, Kurume, Japan.
- KMID: 2173630
- DOI: http://doi.org/10.3802/jgo.2011.22.4.275
Abstract
- Diffusion-weighted imaging (DWI) reflects changes in proton mobility caused by pathological alterations of tissue cellularity, cellular membrane integrity, extracellular space perfusion, and fluid viscosity. Functional imaging is becoming increasingly important in the evaluation of cancer patients because of the limitations of morphologic imaging. DWI is being applied to the detection and characterization of tumors and the evaluation of treatment response in patients with cancer. The advantages of DWI include its cost-effectiveness and brevity of execution, its complete noninvasiveness, its lack of ionizing radiation, and the fact that it does not require injection of contrast material, thus enabling its use in patients with renal dysfunction. In this article, we describe the clinical application of DWI to gynecological disorders and its diagnostic efficacy therein.
Keyword
MeSH Terms
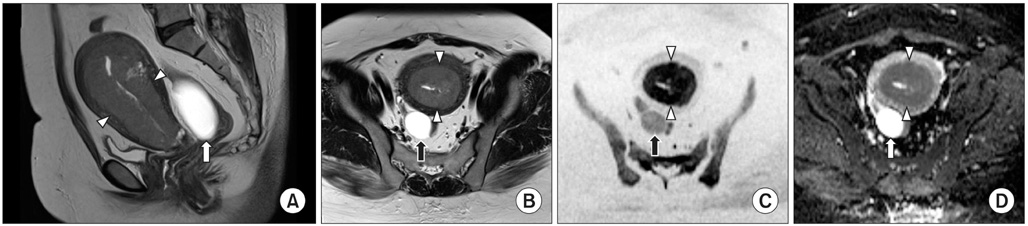
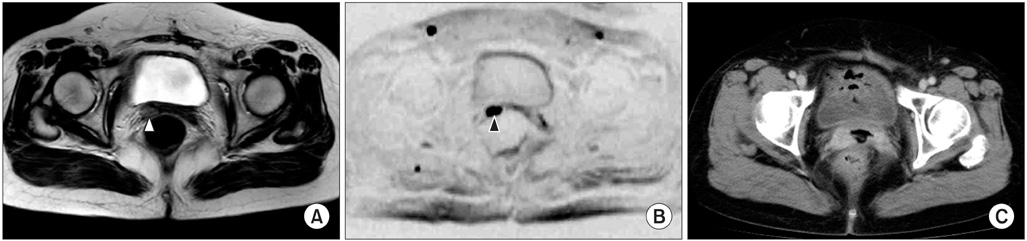
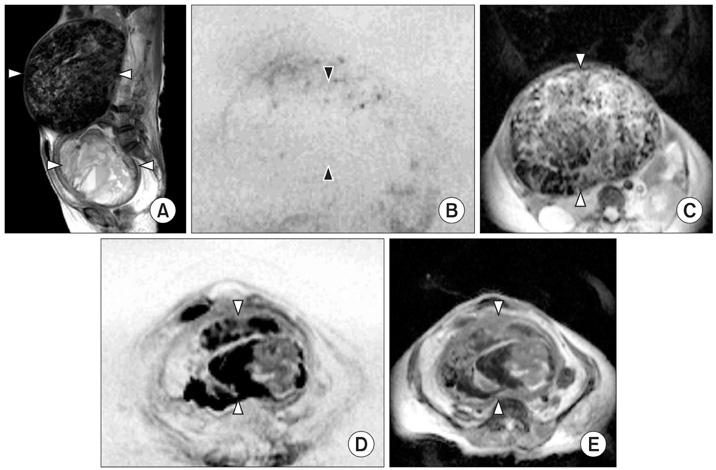
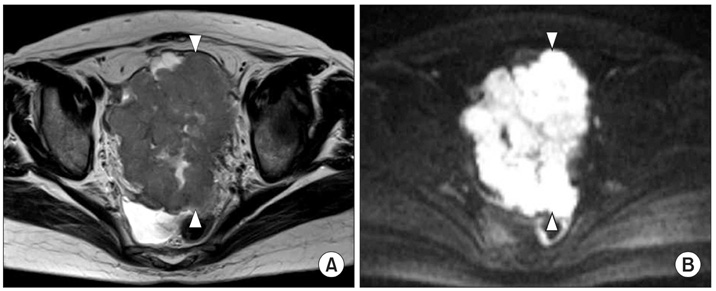
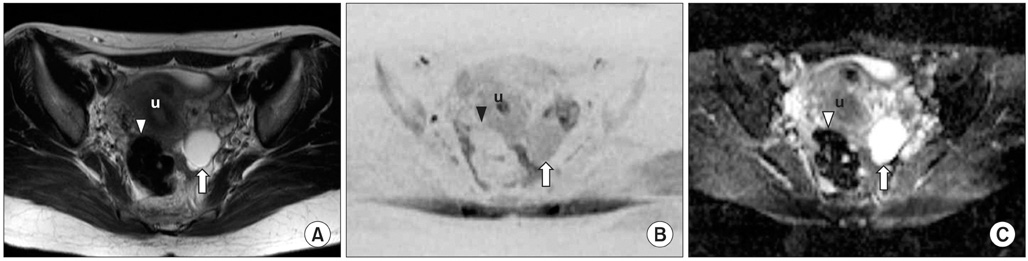
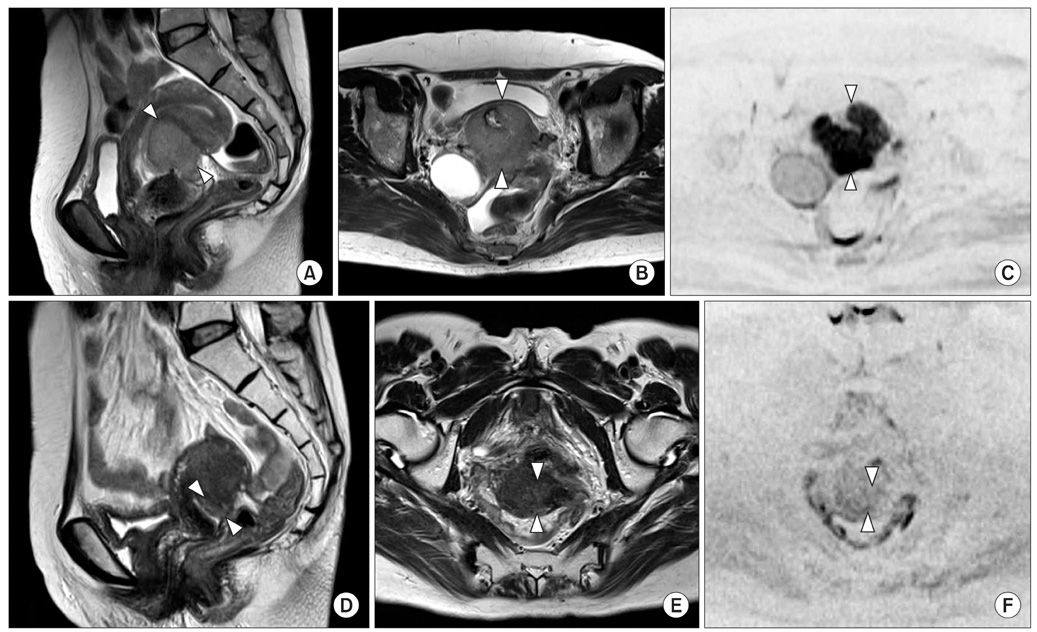
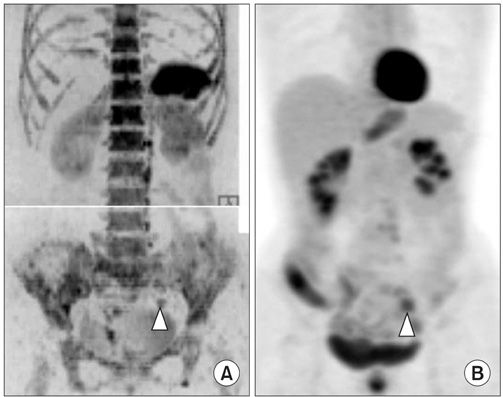
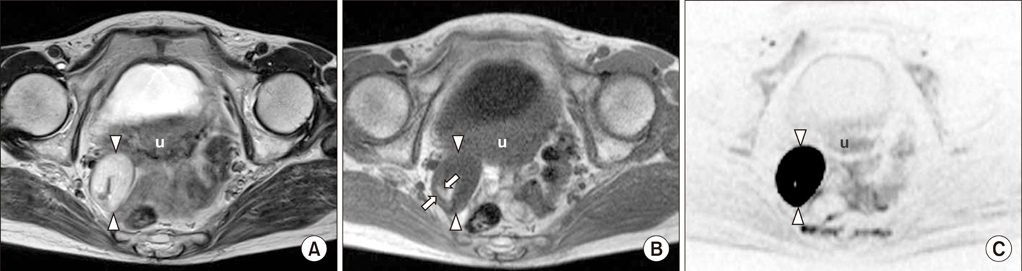
Figure
Reference
-
1. Low RN. Diffusion-weighted MR imaging for whole body metastatic disease and lymphadenopathy. Magn Reson Imaging Clin N Am. 2009. 17:245–261.2. Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009. 11:102–125.3. Inada Y, Matsuki M, Nakai G, Tatsugami F, Tanikake M, Narabayashi I, et al. Body diffusion-weighted MR imaging of uterine endometrial cancer: is it helpful in the detection of cancer in nonenhanced MR imaging? Eur J Radiol. 2009. 70:122–127.4. Koyama T, Togashi K. Functional MR imaging of the female pelvis. J Magn Reson Imaging. 2007. 25:1101–1112.5. Van Beers BE, Vilgrain V. Biomarkers in abdominal imaging. Abdom Imaging. 2009. 34:663–667.6. Kilickesmez O, Bayramoglu S, Inci E, Cimilli T, Kayhan A. Quantitative diffusion-weighted magnetic resonance imaging of normal and diseased uterine zones. Acta Radiol. 2009. 50:340–347.7. Whittaker CS, Coady A, Culver L, Rustin G, Padwick M, Padhani AR. Diffusion-weighted MR imaging of female pelvic tumors: a pictorial review. Radiographics. 2009. 29:759–774.8. Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007. 188:1622–1635.9. Kallehauge JF, Tanderup K, Haack S, Nielsen T, Muren LP, Fokdal L, et al. Apparent Diffusion Coefficient (ADC) as a quantitative parameterin diffusion weighted MR imaging in gynecologic cancer: Dependence on b-values used. Acta Oncol. 2010. 49:1017–1022.10. Koh DM, Takahara T, Imai Y, Collins DJ. Practical aspects of assessing tumors using clinical diffusion-weighted imaging in the body. Magn Reson Med Sci. 2007. 6:211–224.11. Fujii S, Matsusue E, Kigawa J, Sato S, Kanasaki Y, Nakanishi J, et al. Diagnostic accuracy of the apparent diffusion coefficient in differentiating benign from malignant uterine endometrial cavity lesions: initial results. Eur Radiol. 2008. 18:384–389.12. Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, et al. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008. 246:812–822.13. Takeuchi M, Matsuzaki K, Kubo H, Nishitani H. Diffusionweighted magnetic resonance imaging of urinary epithelial cancer with upper urinary tract obstruction: preliminary results. Acta Radiol. 2008. 49:1195–1199.14. Paudyal B, Paudyal P, Tsushima Y, Oriuchi N, Amanuma M, Miyazaki M, et al. The role of the ADC value in the characterisation of renal carcinoma by diffusion-weighted MRI. Br J Radiol. 2010. 83:336–343.15. Kilickesmez O, Atilla S, Soylu A, Tasdelen N, Bayramoglu S, Cimilli T, et al. Diffusion-weighted imaging of the rectosigmoid colon: preliminary findings. J Comput Assist Tomogr. 2009. 33:863–866.16. Rao SX, Zeng MS, Chen CZ, Li RC, Zhang SJ, Xu JM, et al. The value of diffusion-weighted imaging in combination with T2-weighted imaging for rectal cancer detection. Eur J Radiol. 2008. 65:299–303.17. Hosonuma T, Tozaki M, Ichiba N, Sakuma T, Hayashi D, Yanaga K, et al. Clinical usefulness of diffusion-weighted imaging using low and high b-values to detect rectal cancer. Magn Reson Med Sci. 2006. 5:173–177.18. Wang Y, Chen ZE, Nikolaidis P, McCarthy RJ, Merrick L, Sternick LA, et al. Diffusion-weighted magnetic resonance imaging of pancreatic adenocarcinomas: association with histopathology and tumor grade. J Magn Reson Imaging. 2011. 33:136–142.19. Kamisawa T, Takuma K, Anjiki H, Egawa N, Hata T, Kurata M, et al. Differentiation of autoimmune pancreatitis from pancreatic cancer by diffusion-weighted MRI. Am J Gastroenterol. 2010. 105:1870–1875.20. Lemke A, Laun FB, Klauss M, Re TJ, Simon D, Delorme S, et al. Differentiation of pancreas carcinoma from healthy pancreatic tissue using multiple b-values: comparison of apparent diffusion coefficient and intravoxel incoherent motion derived parameters. Invest Radiol. 2009. 44:769–775.21. Kartalis N, Lindholm TL, Aspelin P, Permert J, Albiin N. Diffusion-weighted magnetic resonance imaging of pancreas tumours. Eur Radiol. 2009. 19:1981–1990.22. Fattahi R, Balci NC, Perman WH, Hsueh EC, Alkaade S, Havlioglu N, et al. Pancreatic diffusion-weighted imaging (DWI): comparison between mass-forming focal pancreatitis (FP), pancreatic cancer (PC), and normal pancreas. J Magn Reson Imaging. 2009. 29:350–356.23. Ichikawa T, Erturk SM, Motosugi U, Sou H, Iino H, Araki T, et al. High-b value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: preliminary results. AJR Am J Roentgenol. 2007. 188:409–414.24. Rosenkrantz AB, Mannelli L, Kong X, Niver BE, Berkman DS, Babb JS, et al. Prostate cancer: utility of fusion of T2-weighted and high b-value diffusion-weighted images for peripheral zone tumor detection and localization. J Magn Reson Imaging. 2011. 34:95–100.25. Rosenkrantz AB, Kong X, Niver BE, Berkman DS, Melamed J, Babb JS, et al. Prostate cancer: comparison of tumor visibility on trace diffusion-weighted images and the apparent diffusion coefficient map. AJR Am J Roentgenol. 2011. 196:123–129.26. Iwazawa J, Mitani T, Sassa S, Ohue S. Prostate cancer detection with MRI: is dynamic contrast-enhanced imaging necessary in addition to diffusion-weighted imaging? Diagn Interv Radiol. 2011. 17:243–248.27. Katahira K, Takahara T, Kwee TC, Oda S, Suzuki Y, Morishita S, et al. Ultra-high-b-value diffusion-weighted MR imaging for the detection of prostate cancer: evaluation in 201 cases with histopathological correlation. Eur Radiol. 2011. 21:188–196.28. Kajihara H, Hayashida Y, Murakami R, Katahira K, Nishimura R, Hamada Y, et al. Usefulness of diffusion-weighted imaging in the localization of prostate cancer. Int J Radiat Oncol Biol Phys. 2009. 74:399–403.29. Tamada T, Sone T, Jo Y, Toshimitsu S, Yamashita T, Yamamoto A, et al. Apparent diffusion coefficient values in peripheral and transition zones of the prostate: comparison between normal and malignant prostatic tissues and correlation with histologic grade. J Magn Reson Imaging. 2008. 28:720–726.30. Ren J, Huan Y, Wang H, Zhao H, Ge Y, Chang Y, et al. Diffusion-weighted imaging in normal prostate and differential diagnosis of prostate diseases. Abdom Imaging. 2008. 33:724–728.31. Kim CK, Park BK, Lee HM, Kwon GY. Value of diffusionweighted imaging for the prediction of prostate cancer location at 3T using a phased-array coil: preliminary results. Invest Radiol. 2007. 42:842–847.32. Haider MA, van der Kwast TH, Tanguay J, Evans AJ, Hashmi AT, Lockwood G, et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol. 2007. 189:323–328.33. Kim CK, Park BK, Han JJ, Kang TW, Lee HM. Diffusionweighted imaging of the prostate at 3 T for differentiation of malignant and benign tissue in transition and peripheral zones: preliminary results. J Comput Assist Tomogr. 2007. 31:449–454.34. Pickles MD, Gibbs P, Sreenivas M, Turnbull LW. Diffusionweighted imaging of normal and malignant prostate tissue at 3.0T. J Magn Reson Imaging. 2006. 23:130–134.35. Nasu K, Kuroki Y, Kuroki S, Murakami K, Nawano S, Moriyama N. Diffusion-weighted single shot echo planar imaging of colorectal cancer using a sensitivity-encoding technique. Jpn J Clin Oncol. 2004. 34:620–626.36. Hosseinzadeh K, Schwarz SD. Endorectal diffusionweighted imaging in prostate cancer to differentiate malignant and benign peripheral zone tissue. J Magn Reson Imaging. 2004. 20:654–661.37. Piana G, Trinquart L, Meskine N, Barrau V, Beers BV, Vilgrain V. New MR imaging criteria with a diffusion-weighted sequence for the diagnosis of hepatocellular carcinoma in chronic liver diseases. J Hepatol. 2011. 55:126–132.38. Xu PJ, Yan FH, Wang JH, Shan Y, Ji Y, Chen CZ. Contribution of diffusion-weighted magnetic resonance imaging in the characterization of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver. J Comput Assist Tomogr. 2010. 34:506–512.39. Xu PJ, Yan FH, Wang JH, Lin J, Ji Y. Added value of breathhold diffusion-weighted MRI in detection of small hepatocellular carcinoma lesions compared with dynamic contrastenhanced MRI alone using receiver operating characteristic curve analysis. J Magn Reson Imaging. 2009. 29:341–349.40. Zhang Y, Zhao J, Guo D, Zhong W, Ran L. Evaluation of short-term response of high intensity focused ultrasound ablation for primary hepatic carcinoma: utility of contrastenhanced MRI and diffusion-weighted imaging. Eur J Radiol. 2011. 79:347–352.41. Levy A, Caramella C, Chargari C, Medjhoul A, Rey A, Zareski E, et al. Accuracy of diffusion-weighted echoplanar MR imaging and ADC mapping in the evaluation of residual cervical carcinoma after radiation therapy. Gynecol Oncol. 2011. 123:110–115.42. Lambregts DM, Vandecaveye V, Barbaro B, Bakers FC, Lambrecht M, Maas M, et al. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol. 2011. 18:2224–2231.43. Song I, Kim CK, Park BK, Park W. Assessment of response to radiotherapy for prostate cancer: value of diffusionweighted MRI at 3 T. AJR Am J Roentgenol. 2010. 194:W477–W482.44. Yoshida S, Koga F, Kawakami S, Ishii C, Tanaka H, Numao N, et al. Initial experience of diffusion-weighted magnetic resonance imaging to assess therapeutic response to induction chemoradiotherapy against muscle-invasive bladder cancer. Urology. 2010. 75:387–391.45. Eccles CL, Haider EA, Haider MA, Fung S, Lockwood G, Dawson LA. Change in diffusion weighted MRI during liver cancer radiotherapy: preliminary observations. Acta Oncol. 2009. 48:1034–1043.46. Naganawa S, Sato C, Kumada H, Ishigaki T, Miura S, Takizawa O. Apparent diffusion coefficient in cervical cancer of the uterus: comparison with the normal uterine cervix. Eur Radiol. 2005. 15:71–78.47. Namimoto T, Awai K, Nakaura T, Yanaga Y, Hirai T, Yamashita Y. Role of diffusion-weighted imaging in the diagnosis of gynecological diseases. Eur Radiol. 2009. 19:745–760.48. Sugita R, Ito K, Fujita N, Takahashi S. Diffusion-weighted MRI in abdominal oncology: clinical applications. World J Gastroenterol. 2010. 16:832–836.49. Tamai K, Koyama T, Saga T, Umeoka S, Mikami Y, Fujii S, et al. Diffusion-weighted MR imaging of uterine endometrial cancer. J Magn Reson Imaging. 2007. 26:682–687.50. Kyriazi S, Collins DJ, Morgan VA, Giles SL, deSouza NM. Diffusion-weighted imaging of peritoneal disease for noninvasive staging of advanced ovarian cancer. Radiographics. 2010. 30:1269–1285.51. Kido A, Kataoka M, Koyama T, Yamamoto A, Saga T, Togashi K. Changes in apparent diffusion coefficients in the normal uterus during different phases of the menstrual cycle. Br J Radiol. 2010. 83:524–528.52. Feuerlein S, Pauls S, Juchems MS, Stuber T, Hoffmann MH, Brambs HJ, et al. Pitfalls in abdominal diffusion-weighted imaging: how predictive is restricted water diffusion for malignancy. AJR Am J Roentgenol. 2009. 193:1070–1076.53. Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics. 2009. 29:1797–1810.54. McVeigh PZ, Syed AM, Milosevic M, Fyles A, Haider MA. Diffusion-weighted MRI in cervical cancer. Eur Radiol. 2008. 18:1058–1064.55. Liu Y, Bai R, Sun H, Liu H, Wang D. Diffusion-weighted magnetic resonance imaging of uterine cervical cancer. J Comput Assist Tomogr. 2009. 33:858–862.56. Fujii S, Matsusue E, Kanasaki Y, Kanamori Y, Nakanishi J, Sugihara S, et al. Detection of peritoneal dissemination in gynecological malignancy: evaluation by diffusionweighted MR imaging. Eur Radiol. 2008. 18:18–23.57. Low RN, Sebrechts CP, Barone RM, Muller W. Diffusionweighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings--a feasibility study. AJR Am J Roentgenol. 2009. 193:461–470.58. Shinya S, Sasaki T, Nakagawa Y, Guiquing Z, Yamamoto F, Yamashita Y. The usefulness of diffusion-weighted imaging (DWI) for the detection of gastric cancer. Hepatogastroenterology. 2007. 54:1378–1381.59. Roy C, Bierry G, Matau A, Bazille G, Pasquali R. Value of diffusion-weighted imaging to detect small malignant pelvic lymph nodes at 3 T. Eur Radiol. 2010. 20:1803–1811.60. Mir N, Sohaib SA, Collins D, Koh DM. Fusion of high b-value diffusion-weighted and T2-weighted MR images improves identification of lymph nodes in the pelvis. J Med Imaging Radiat Oncol. 2010. 54:358–364.61. Kim JK, Kim KA, Park BW, Kim N, Cho KS. Feasibility of diffusion-weighted imaging in the differentiation of metastatic from nonmetastatic lymph nodes: early experience. J Magn Reson Imaging. 2008. 28:714–719.62. Chen YB, Liao J, Xie R, Chen GL, Chen G. Discrimination of metastatic from hyperplastic pelvic lymph nodes in patients with cervical cancer by diffusion-weighted magnetic resonance imaging. Abdom Imaging. 2011. 36:102–109.63. Rockall AG, Sohaib SA, Harisinghani MG, Babar SA, Singh N, Jeyarajah AR, et al. Diagnostic performance of nanoparticle-enhanced magnetic resonance imaging in the diagnosis of lymph node metastases in patients with endometrial and cervical cancer. J Clin Oncol. 2005. 23:2813–2821.64. Torabi M, Aquino SL, Harisinghani MG. Current concepts in lymph node imaging. J Nucl Med. 2004. 45:1509–1518.65. Lin G, Ho KC, Wang JJ, Ng KK, Wai YY, Chen YT, et al. Detection of lymph node metastasis in cervical and uterine cancers by diffusion-weighted magnetic resonance imaging at 3T. J Magn Reson Imaging. 2008. 28:128–135.66. Park SO, Kim JK, Kim KA, Park BW, Kim N, Cho G, et al. Relative apparent diffusion coefficient: determination of reference site and validation of benefit for detecting metastatic lymph nodes in uterine cervical cancer. J Magn Reson Imaging. 2009. 29:383–390.67. Sumi M, Sakihama N, Sumi T, Morikawa M, Uetani M, Kabasawa H, et al. Discrimination of metastatic cervical lymph nodes with diffusion-weighted MR imaging in patients with head and neck cancer. AJNR Am J Neuroradiol. 2003. 24:1627–1634.68. Nakai G, Matsuki M, Inada Y, Tatsugami F, Tanikake M, Narabayashi I, et al. Detection and evaluation of pelvic lymph nodes in patients with gynecologic malignancies using body diffusion-weighted magnetic resonance imaging. J Comput Assist Tomogr. 2008. 32:764–768.69. Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004. 22:275–282.70. Fasih N, Prasad Shanbhogue AK, Macdonald DB, Fraser-Hill MA, Papadatos D, Kielar AZ, et al. Leiomyomas beyond the uterus: unusual locations, rare manifestations. Radiographics. 2008. 28:1931–1948.71. Tamai K, Koyama T, Saga T, Morisawa N, Fujimoto K, Mikami Y, et al. The utility of diffusion-weighted MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol. 2008. 18:723–730.72. Hricak H, Chen M, Coakley FV, Kinkel K, Yu KK, Sica G, et al. Complex adnexal masses: detection and characterization with MR imaging--multivariate analysis. Radiology. 2000. 214:39–46.73. Thomassin-Naggara I, Toussaint I, Perrot N, Rouzier R, Cuenod CA, Bazot M, et al. Characterization of complex adnexal masses: value of adding perfusion- and diffusionweighted MR imaging to conventional MR imaging. Radiology. 2011. 258:793–803.74. Thomassin-Naggara I, Darai E, Cuenod CA, Fournier L, Toussaint I, Marsault C, et al. Contribution of diffusionweighted MR imaging for predicting benignity of complex adnexal masses. Eur Radiol. 2009. 19:1544–1552.75. Takeuchi M, Matsuzaki K, Nishitani H. Diffusionweighted magnetic resonance imaging of ovarian tumors: differentiation of benign and malignant solid components of ovarian masses. J Comput Assist Tomogr. 2010. 34:173–176.76. Nakayama T, Yoshimitsu K, Irie H, Aibe H, Tajima T, Nishie A, et al. Diffusion-weighted echo-planar MR imaging and ADC mapping in the differential diagnosis of ovarian cystic masses: usefulness of detecting keratinoid substances in mature cystic teratomas. J Magn Reson Imaging. 2005. 22:271–278.77. Katayama M, Masui T, Kobayashi S, Ito T, Sakahara H, Nozaki A, et al. Diffusion-weighted echo planar imaging of ovarian tumors: is it useful to measure apparent diffusion coefficients? J Comput Assist Tomogr. 2002. 26:250–256.78. Hamstra DA, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: a biomarker for treatment response in oncology. J Clin Oncol. 2007. 25:4104–4109.79. Liu Y, Bai R, Sun H, Liu H, Zhao X, Li Y. Diffusion-weighted imaging in predicting and monitoring the response of uterine cervical cancer to combined chemoradiation. Clin Radiol. 2009. 64:1067–1074.80. Kyriazi S, Collins DJ, Messiou C, Pennert K, Davidson RL, Giles SL, et al. Metastatic ovarian and primary peritoneal cancer: assessing chemotherapy response with diffusionweighted MR imaging--value of histogram analysis of apparent diffusion coefficients. Radiology. 2011. 261:182–192.81. Armitage PA, Schwindack C, Bastin ME, Whittle IR. Quantitative assessment of intracranial tumor response to dexamethasone using diffusion, perfusion and permeability magnetic resonance imaging. Magn Reson Imaging. 2007. 25:303–310.82. Rhee TK, Naik NK, Deng J, Atassi B, Mulcahy MF, Kulik LM, et al. Tumor response after yttrium-90 radioembolization for hepatocellular carcinoma: comparison of diffusionweighted functional MR imaging with anatomic MR imaging. J Vasc Interv Radiol. 2008. 19:1180–1186.83. Sharma U, Danishad KK, Seenu V, Jagannathan NR. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009. 22:104–113.84. Chen CY, Li CW, Kuo YT, Jaw TS, Wu DK, Jao JC, et al. Early response of hepatocellular carcinoma to transcatheter arterial chemoembolization: choline levels and MR diffusion constants--initial experience. Radiology. 2006. 239:448–456.85. Cui Y, Zhang XP, Sun YS, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008. 248:894–900.86. Harry VN, Semple SI, Gilbert FJ, Parkin DE. Diffusionweighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol Oncol. 2008. 111:213–220.87. Koh DM, Scurr E, Collins D, Kanber B, Norman A, Leach MO, et al. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol. 2007. 188:1001–1008.88. DeVries AF, Kremser C, Hein PA, Griebel J, Krezcy A, Ofner D, et al. Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int J Radiat Oncol Biol Phys. 2003. 56:958–965.89. Dzik-Jurasz A, Domenig C, George M, Wolber J, Padhani A, Brown G, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002. 360:307–308.90. Kwee TC, Takahara T, Ochiai R, Nievelstein RA, Luijten PR. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol. 2008. 18:1937–1952.91. Ho KC, Lin G, Wang JJ, Lai CH, Chang CJ, Yen TC. Correlation of apparent diffusion coefficients measured by 3T diffusion-weighted MRI and SUV from FDG PET/CT in primary cervical cancer. Eur J Nucl Med Mol Imaging. 2009. 36:200–208.92. Komori T, Narabayashi I, Matsumura K, Matsuki M, Akagi H, Ogura Y, et al. 2-[Fluorine-18]-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography versus whole-body diffusion-weighted MRI for detection of malignant lesions: initial experience. Ann Nucl Med. 2007. 21:209–215.93. Satoh Y, Ichikawa T, Motosugi U, Kimura K, Sou H, Sano K, et al. Diagnosis of peritoneal dissemination: comparison of 18F-FDG PET/CT, diffusion-weighted MRI, and contrastenhanced MDCT. AJR Am J Roentgenol. 2011. 196:447–453.94. Takeshita T, Ninoi T, Doh K, Hashimoto S, Inoue Y. Diffusion-weighted magnetic resonance imaging in tubo-ovarian abscess: a case report. Osaka City Med J. 2009. 55:109–114.95. Moteki T, Ishizaka H. Diffusion-weighted EPI of cystic ovarian lesions: evaluation of cystic contents using apparent diffusion coefficients. J Magn Reson Imaging. 2000. 12:1014–1019.96. Busard MP, Mijatovic V, van Kuijk C, Pieters-van den Bos IC, Hompes PG, van Waesberghe JH. Magnetic resonance imaging in the evaluation of (deep infiltrating) endometriosis: the value of diffusion-weighted imaging. J Magn Reson Imaging. 2010. 31:1117–1123.97. Oussalah A, Laurent V, Bruot O, Bressenot A, Bigard MA, Regent D, et al. Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut. 2010. 59:1056–1065.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MR Spectroscopy and Diffusion Weighted Imaging Findings of Primary Non-Hodgkin Lymphoma of the Breast: Two Case Reports
- MR Imaging of Skeletal Muscle Injury in Rabbit: Comparison bet ween Diffusion and T2-weighted MR Images
- Diffusion-Weighted MR Imaging in Acute Wernicke's Encephalopathy Associated with Pseudomembranous Colitis: A Case Report and Review of the Literature
- Diffusion Weighted MRI Findings in Two Patients with Hypoxic Brain Damage
- Diffusion-Weighted MR Imaging in Biopsy-Proven Creutzfeldt-Jakob Disease