J Cerebrovasc Endovasc Neurosurg.
2013 Mar;15(1):20-25. 10.7461/jcen.2013.15.1.20.
An Efficient Microvascular Anastomosis Training Model Based on Chicken Wings and Simple Instruments
- Affiliations
-
- 1Department of Neurosurgery, Busan Paik Hospital, School of Medicine, Inje University, Busan, Korea. kimst015@hanmail.net
- KMID: 2172026
- DOI: http://doi.org/10.7461/jcen.2013.15.1.20
Abstract
OBJECTIVE
The aim of this study is to introduce a microvascular training model based on use of materials that can be easily obtained from the daily surroundings.
METHODS
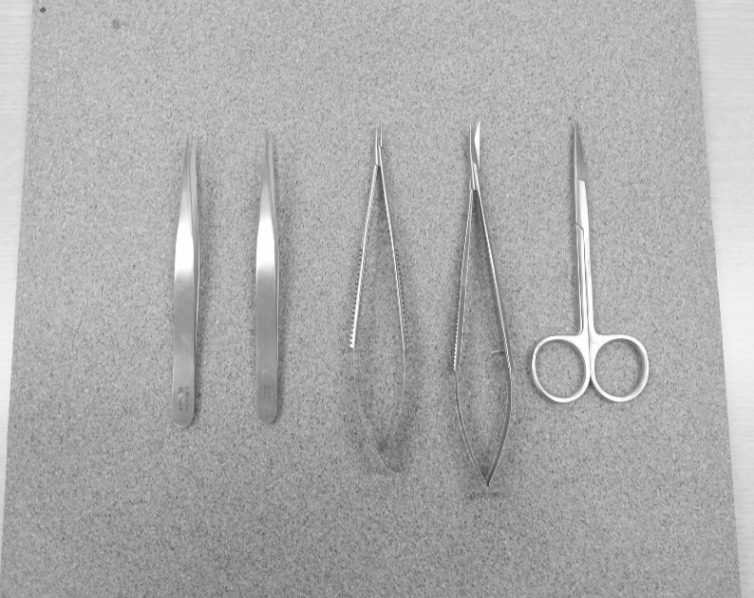
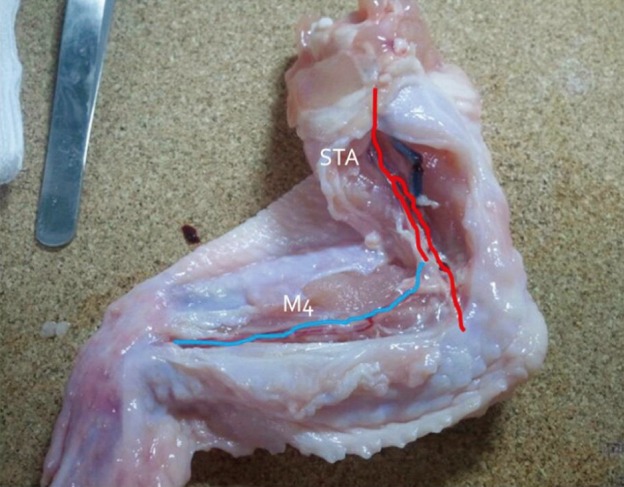
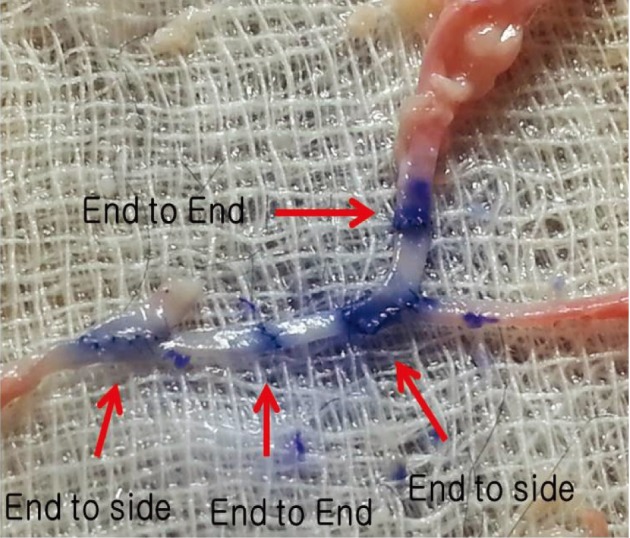
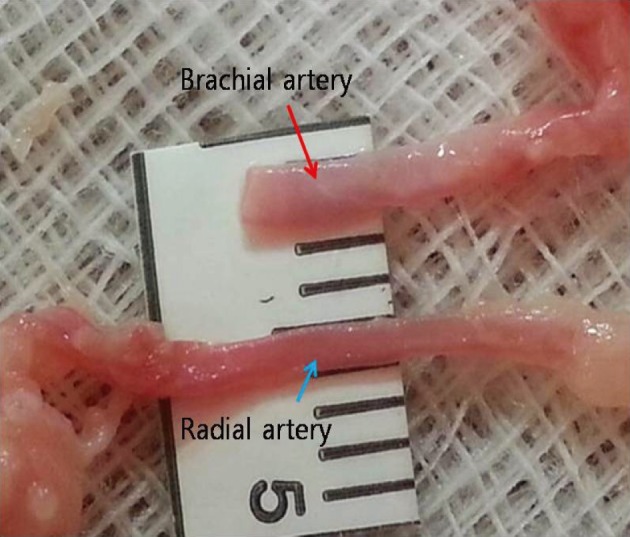
Simple microinstruments and a medical school laboratory microscope were used for anastomosis training. Chicken blood vessels were used as a material for this study. A long segment of blood vessel from the proximal brachial artery to the distal radial artery was used for training. End-to-side anastomosis was practiced first, and the training continued with end-to-end anastomosis of the appropriate segments.
RESULTS
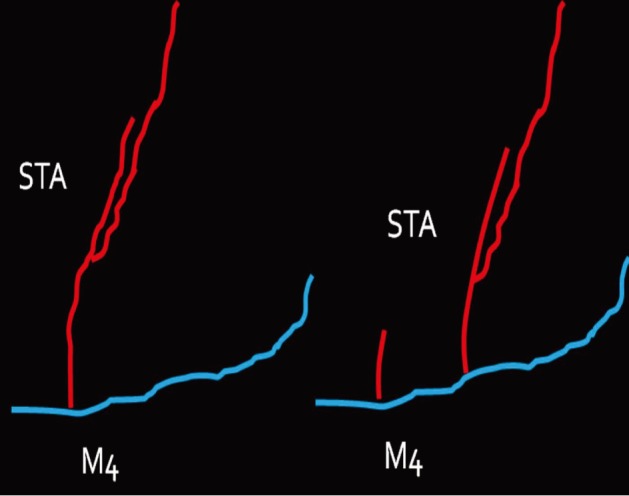
The instruments used for setting up this model were simple and easy to use; therefore, the time required for preparation of the materials and dissection of the chicken wings was only approximately five to ten minutes. The characteristics of 20 chicken wings were analyzed. The length of the brachial artery to the radial artery was 8 - 10 cm. The average diameter of the brachial artery was 1.3 mm +/- 0.2 mm and that of the radial artery was 1.0 mm +/- 0.2 mm. Taking advantage of these characteristics, the proximal brachial artery was grafted to the radial artery for practice of end-to-side anastomosis.
CONCLUSIONS
This study suggests an effective and feasible method for microvascular anastomosis training using chicken wing arteries and simple microinstruments. This model may simulate the conditions of a superficial temporal artery to middle cerebral artery anastomosis surgery.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A mathematical approach to microvascular anastomosis for beginners and the effectiveness of chicken legs and partial meat from chicken legs and chicken wings as a training model
Dong Young Kim, Seung Bum Chae
Arch Hand Microsurg. 2024;29(4):269-275. doi: 10.12790/ahm.24.0036.
Reference
-
1. Abla AA, Uschold T, Preul MC, Zabramski JM. Comparative use of turkey and chicken wing brachial artery models for microvascular anastomosis training. J Neurosurg. 2011; 115(6):1231–1235. PMID: 21962125.
Article2. Douglas HE, Mackay IR. Microvascular surgical training models. J Plast Reconstr Aesthet Surg. 2011; 8. 64(8):e210–e212. PMID: 21277267.
Article3. Hwang G, Oh CW, Bang JS, Jung CK, Kwon OK, Kim JE, et al. Superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke and stroke in progress. Neurosurgery. 2011; 3. 68(3):723–729. discussion 729-30. PMID: 21311299.
Article4. Hwang G, Oh CW, Park SQ, Sheen SH, Bang JS, Kang HS. Comparison of different microanastomosis training models: model accuracy and practicality. J Korean Neurosurg Soc. 2010; 4. 47(4):287–290. PMID: 20461170.5. Hino A. Training in microvascular surgery using a chicken wing artery. Neurosurgery. 2003; 6. 52(6):1495–1497. discussion 1497-8. PMID: 12762899.
Article6. Hong JW, Kim YS, Lee WJ, Hong HJ, Roh TS, Song SY. Evaluation of the efficacy of microsurgical practice through time factor added protocol: microsurgical training using nonvital material. J Craniofac Surg. 2010; 5. 21(3):876–881. PMID: 20485073.7. Inoue T, Tsutsumi K, Adachi S, Tanaka S, Saito K, Kunii N. Effectiveness of suturing training with 10-0 nylon under fixed and maximum magnification (× 20) using desk type microscope. Surg Neurol. 2006; 8. 66(2):183–187. PMID: 16876622.8. JET Study group. [Japanese EC-IC Trial (JET Study). Study design and interim analysis]. Surg Cereb Stroke. 2002; 30(2):97–100. Japanese.9. Kadri PA, Krisht AF, Gandhi GK. An anatomic mathematical measurement to find an adequate recipient M4 branch for superficial temporal artery to middle cerebral artery bypass surgery. Neurosurgery. 2007; 9. 61(3 Suppl):74–78. discussion 78. PMID: 17876234.
Article10. Krishnan KG, Dramm P, Schackert G. Simple and viable in vitro perfusion model for training microvascular anastomoses. Microsurgery. 2004; 24(4):335–338. PMID: 15274194.
Article11. Lannon DA, Atkins JA, Butler PE. Non-vital, prosthetic, and virtual reality models of microsurgical training. Microsurgery. 2001; 21(8):389–393. PMID: 11757067.
Article12. Lascar I, Totir D, Cinca A, Cortan S, Stefanescu A, Bratianu R, et al. Training program and learning curve in experimental microsurgery during the residency in plastic surgery. Microsurgery. 2007; 27(4):263–267. PMID: 17477411.
Article13. Levinsohn EM, Packard DS Jr, West EM, Hootnick DR. Arterial anatomy of chicken embryo and hatchling. Am J Anat. 1984; 4. 169(4):377–405. PMID: 6731332.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A mathematical approach to microvascular anastomosis for beginners and the effectiveness of chicken legs and partial meat from chicken legs and chicken wings as a training model
- Characteristics of Training Materials for Successful Microvascular Anastomosis and Preclinical Assessment of The Surgical Skills
- Comprehensive Analysis of Chicken Vessels as Microvascular Anastomosis Training Model
- Training of Microanastomosis with Chicken Wing Brachial Artery
- The Chicken Thigh Adductor Profundus Free Muscle Flap: A Novel Validated Non-Living Microsurgery Simulation Training Model