Ann Dermatol.
2011 Aug;23(3):321-328. 10.5021/ad.2011.23.3.321.
Epidemiologic Study of Malassezia Yeasts in Acne Patients by Analysis of 26S rDNA PCR-RFLP
- Affiliations
-
- 1Department of Dermatology, Konkuk University School of Medicine, Seoul, Korea. 20050078@kuh.ac.kr
- KMID: 2171923
- DOI: http://doi.org/10.5021/ad.2011.23.3.321
Abstract
- BACKGROUND
Although acne is a common follicular inflammatory dermatosis, studies of the relationship between Malassezia yeasts and acne have rarely been conducted.
OBJECTIVE
We sought to identify Malassezia yeasts from acne patients and establish a relationship between specific types of species of Malassezia and acne.
METHODS
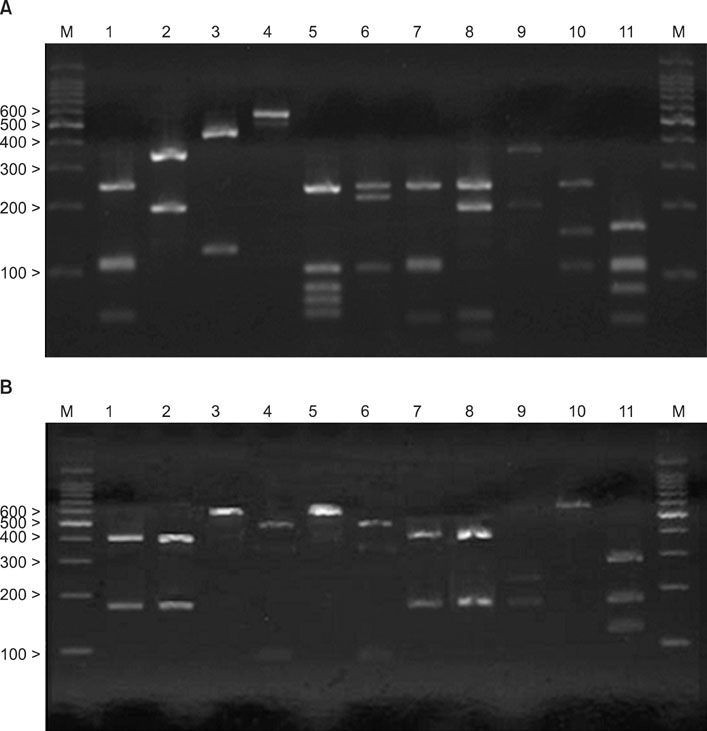
Sixty acne patients were enrolled. Each strain obtained was identified as one of eleven species by 26S rDNA PCR-RFLP. We then compared these data with those of age- and sex-matched healthy subjects.
RESULTS
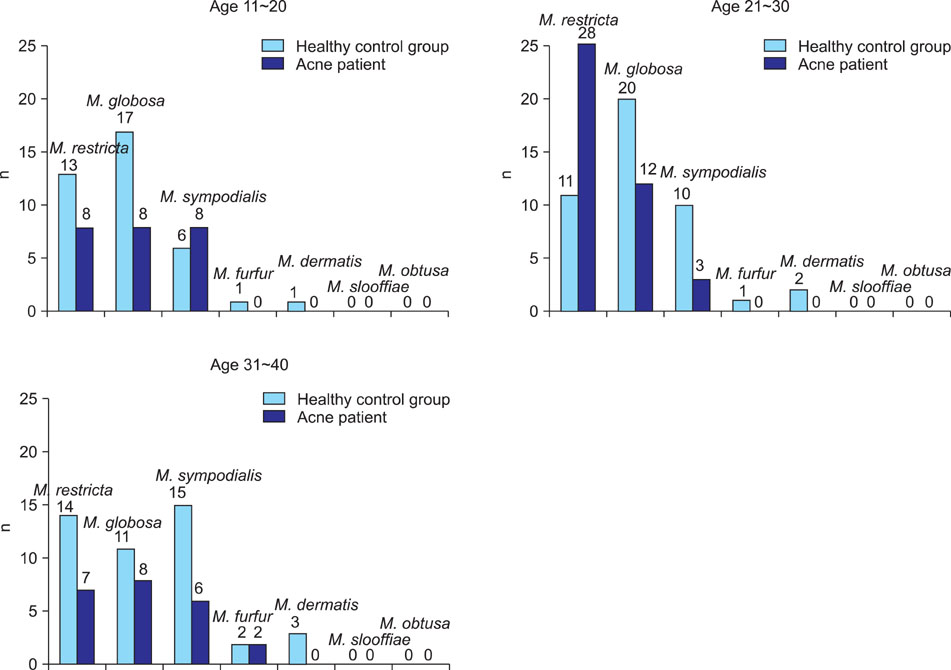
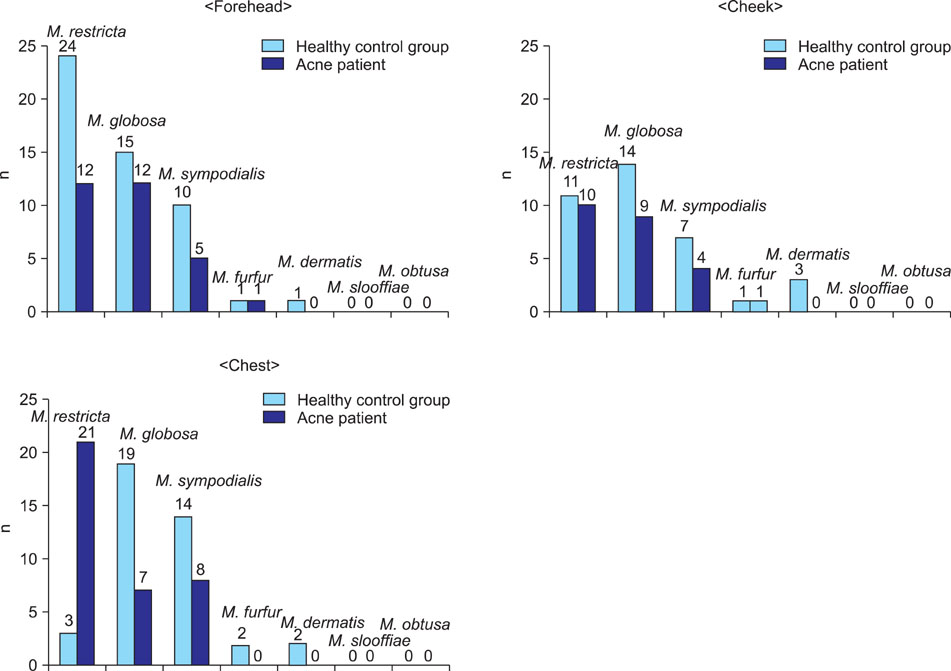
Growth of Malassezia was evident in fewer patients with acne (50%) in comparison to controls (70.6%). M. restricta was dominant in patients with acne (23.9%), whereas M. globosa was most common (26.7%) in healthy controls. In the patients group, the rate was the highest (71.7%) in the twenties and, in terms of body site, the rate was the highest (60%) in the chest. In the control group, the rate was the highest (75.0%) in the thirties and in the forehead (85.0%).
CONCLUSION
The detection rate of Malassezia yeasts was conspicuously low in the acne patients group. Statistically significant differences were observed between the patient and the control groups in the twenties and thirties, and in terms of body site, in the forehead and chest.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Progress in Malassezia Research in Korea
Soo Young Kim, Yang Won Lee, Yong Beom Choe, Kyu Joong Ahn
Ann Dermatol. 2015;27(6):647-657. doi: 10.5021/ad.2015.27.6.647.
Reference
-
1. Zaenglein AL, Graber EM, Thiboutot DM, Strauss JS. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Acne vulgaris and acneiform eruptions. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;690–703.2. Youn NH, Cha SH, Park SD. Malassezia yeasts in acne vulgaris. Korean J Dermatol. 2002. 40:1453–1460.3. Leeming JP, Holland KT, Cuncliffe WJ. The microbial colonization of inflamed acne vulgaris lesions. Br J Dermatol. 1988. 118:203–208.
Article4. Janik MP, Heffernan MP. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Yeast infections: Candidiasis, Pityriasis (Tinea) versicolor. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1822–1830.5. Ahn KJ. Taxonomy of the genus Malassezia. Korean J Med Mycol. 1998. 3:81–88.6. Guillot J, Guého E. The diversity of Malassezia yeasts confirmed by rRNA sequence and nuclear DNA comparisons. Antonie Van Leeuwenhoek. 1995. 67:297–314.7. Guillot J, Gueho E, Lesourd M, Midgley G, Chevrier G, Dupont B. Identification of Malassezia species: a practical approach. J Mycol Med. 1996. 6:103–110.8. Yamada Y, Makimura K, Ueda K, Nishiyama Y, Uchida K, Yamaguchi H, et al. DNA base alignment and taxonomic study of genus Malassezia based upon partial sequences of mitochondrial large subunit ribosomal RNA gene. Microbiol Immunol. 2003. 47:475–478.
Article9. Guého E, Midgley G, Guillot J. The genus Malassezia with description of four new species. Antonie Van Leeuwenhoek. 1996. 69:337–355.
Article10. Kim SM, Lim SH, Jung BR, Lee YW, Choe YB, Ahn KJ. The application of colony PCR in the molecular biological analysis of Malassezia yeasts. Korean J Med Mycol. 2007. 12:180–188.11. Sugita T, Takashima M, Shinoda T, Suto H, Unno T, Tsuboi R, et al. New yeast species, Malassezia dermatis, isolated from patients with atopic dermatitis. J Clin Microbiol. 2002. 40:1363–1367.
Article12. Hirai A, Kano R, Makimura K, Duarte ER, Hamdan JS, Lachance MA, et al. Malassezia nana sp. nov., a novel lipid-dependent yeast species isolated from animals. Int J Syst Evol Microbiol. 2004. 54:623–627.
Article13. Sugita T, Takashima M, Kodama M, Tsuboi R, Nishikawa A. Description of a new yeast species, Malassezia japonica, and its detection in patients with atopic dermatitis and healthy subjects. J Clin Microbiol. 2003. 41:4695–4699.
Article14. Sugita T, Tajima M, Takashima M, Amaya M, Saito M, Tsuboi R, et al. A new yeast, Malassezia yamatoensis, isolated from a patient with seborrheic dermatitis, and its distribution in patients and healthy subjects. Microbiol Immunol. 2004. 48:579–583.
Article15. Cabañes FJ, Theelen B, Castellá G, Boekhout T. Two new lipid-dependent Malassezia species from domestic animals. FEMS Yeast Res. 2007. 7:1064–1076.16. Ljubojević S, Skerlev M, Lipozencić J, Basta-Juzbasić A. The role of Malassezia furfur in dermatology. Clin Dermatol. 2002. 20:179–182.17. Kanda N, Tani K, Enomoto U, Nakai K, Watanabe S. The skin fungus-induced Th1- and Th2-related cytokine, chemokine and prostaglandin E2 production in peripheral blood mononuclear cells from patients with atopic dermatitis and psoriasis vulgaris. Clin Exp Allergy. 2002. 32:1243–1250.
Article18. Gupta AK, Batra R, Bluhm R, Boekhout T, Dawson TL Jr. Skin diseases associated with Malassezia species. J Am Acad Dermatol. 2004. 51:785–798.19. Kang SH, Kim HU. The isolation of Malassezia yeasts in the comedones of acne vulgaris. Korean J Med Mycol. 1999. 4:33–39.20. Williamson P, Kligman AM. A new method for the quantitative investigation of cutaneous bacteria. J Invest Dermatol. 1965. 45:498–503.
Article21. Leeming JP, Notman FH, Holland KT. The distribution and ecology of Malassezia furfur and cutaneous bacteria on human skin. J Appl Bacteriol. 1989. 67:47–52.
Article22. Yamada Y, Makimura K, Merhendi H, Ueda K, Nishiyama Y, Yamaguchi H, et al. Comparison of different methods for extraction of mitochondrial DNA from human pathogenic yeasts. Jpn J Infect Dis. 2002. 55:122–125.23. Lee YW, Lim SH, Ahn KJ. The application of 26S rDNA PCR-RFLP in the identification and classification of Malassezia yeast. Korean J Med Mycol. 2006. 11:141–153.24. Toyoda M, Morohashi M. Pathogenesis of acne. Med Electron Microsc. 2001. 34:29–40.
Article25. Seo YJ, Piao YJ, Suhr KB, Lee JH, Park JK. A comparative study on clinical and therapeutic features between Malassezia folliculitis and steroid acne. Korean J Med Mycol. 2001. 6:160–166.26. Ayers K, Sweeney SM, Wiss K. Pityrosporum folliculitis: diagnosis and management in 6 female adolescents with acne vulgaris. Arch Pediatr Adolesc Med. 2005. 159:64–67.27. Yu HJ, Kim YS, Yang HY, Kim JH, Lee SK, Son SJ. The incidences of Malassezia in steroid acne and other acneiform eruptions. Korean J Med Mycol. 1998. 3:24–32.28. Kang MJ, Hahm JH. Comparative study of acne on clinical features and patient understandings in adolescence and post-adolescence. Korean J Dermatol. 2000. 38:589–599.29. Jacinto-Jamora S, Tamesis J, Katigbak ML. Pityrosporum folliculitis in the Philippines: diagnosis, prevalence, and management. J Am Acad Dermatol. 1991. 24:693–696.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Application of 26S rDNA PCR-RFLP in the Identification and Classification of Malassezia Yeast
- Epidemiologic Study of Malassezia Yeasts in Patients with Malassezia Folliculitis by 26S rDNA PCR-RFLP Analysis
- Epidemiologic Study of Malassezia Yeasts in Seborrheic Dermatitis Patients by the Analysis of 26S rDNA PCR-RFLP
- Comparison of Nested PCR and RFLP for Identification and Classification of Malassezia Yeasts from Healthy Human Skin
- Molecular Analysis of Malassezia Microflora on the Skin of the Patients with Atopic Dermatitis