Ewha Med J.
2014 Mar;37(1):60-63. 10.12771/emj.2014.37.1.60.
Chelidonium majus-Induced Acute Hepatitis
- Affiliations
-
- 1Hepatology Center, Bundang Jesaeng General Hospital, Seongnam, Korea. hepayoo@dmc.or.kr
- 2Department of Pathology, Bundang Jesaeng General Hospital, Seongnam, Korea.
- KMID: 2171277
- DOI: http://doi.org/10.12771/emj.2014.37.1.60
Abstract
- The use of traditional folk remedies is increasing throughout Asia. Chelidonium majus, a popular herbal remedy, is used to treat abdominal pain caused by various gastrointestinal disorders, including gastric ulcer, gastritis, and biliary tract disease, because of its morphine-like effect. We encountered a 62-year-old woman with acute hepatitis, in which C. majus was suspected to be the etiological factor. The patient had taken high dose of C. majus extract for the preceding 60 days. The clinical context and the temporal association between the start of the herbal medicine treatment and her liver injury allowed us to attribute a causative role to C. majus. The diagnosis was confirmed by liver biopsy and the Council for International Organizations of Medical Sciences/Roussel Uclaf Causality Assessment Method (CIOMS/RUCAM) scale. After C. majus was discontinued, the liver function was restored to normal. In conclusion, because the use of phytotherapy is increasing, we wish to raise awareness of the potential adverse effects of C. majus.
MeSH Terms
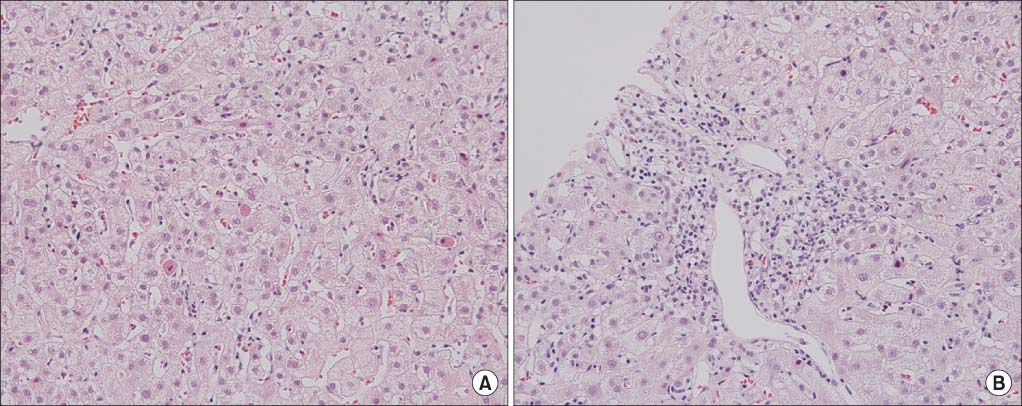
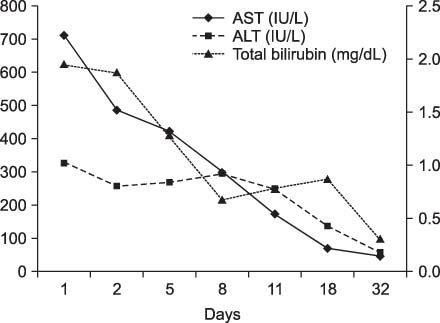
Figure
Reference
-
1. Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol. 2012; 18:249–257.2. Kang SH, Kim JI, Jeong KH, Ko KH, Ko PG, Hwang SW, et al. Clinical characteristics of 159 cases of acute toxic hepatitis. Korean J Hepatol. 2008; 14:483–492.3. Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002; 137:947–954.4. Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, Kim YS, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012; 107:1380–1387.5. Pieroni A, Dibra B, Grishaj G, Grishaj I, Gjon Maçai S. Traditional phytotherapy of the Albanians of Lepushe, Northern Albanian Alps. Fitoterapia. 2005; 76:379–399.6. Etxenagusia MA, Anda M, Gonzalez-Mahave I, Fernandez E, Fernandez de Corres L. Contact dermatitis from Chelidonium majus (greater celandine). Contact Dermatitis. 2000; 43:47.7. Benninger J, Schneider HT, Schuppan D, Kirchner T, Hahn EG. Acute hepatitis induced by greater celandine (Chelidonium majus). Gastroenterology. 1999; 117:1234–1237.8. Stickel F, Pöschl G, Seitz HK, Waldherr R, Hahn EG, Schuppan D. Acute hepatitis induced by greater celandine (Chelidonium majus). Scand J Gastroenterol. 2003; 38:565–568.9. Watkins PB, Seeff LB. Drug-induced liver injury: summary of a single topic clinical research conference. Hepatology. 2006; 43:618–631.10. Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993; 46:1323–1330.11. Boyer T, Manns MP, Sanyal AJ. Hepatotoxicity of herbal preparations. In : Boyer T, Manns MP, Sanyal AJ, editors. Zakim and Boyer's hepatology: a textbook of liver disease. 6th ed. Philadelphia, PA: Elsevier Saunders;2011. p. 462–475.12. Colombo ML, Bosisio E. Pharmacological activities of Chelidonium majus L. (Papaveraceae). Pharmacol Res. 1996; 33:127–134.13. Gilca M, Gaman L, Panait E, Stoian I, Atanasiu V. Chelidonium majus: an integrative review: traditional knowledge versus modern findings. Forsch Komplementmed. 2010; 17:241–248.14. Jeng JH, Wu HL, Lin BR, Lan WH, Chang HH, Ho YS, et al. Antiplatelet effect of sanguinarine is correlated to calcium mobilization, thromboxane and cAMP production. Atherosclerosis. 2007; 191:250–258.15. Andrade RJ, Robles M, Fernandez-Castaner A, Lopez-Ortega S, Lopez-Vega MC, Lucena MI. Assessment of drug-induced hepatotoxicity in clinical practice: a challenge for gastroenterologists. World J Gastroenterol. 2007; 13:329–340.16. Danan G, Benichou C. Causality assessment of adverse reactions to drugs I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993; 46:1323–1330.17. Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993; 46:1331–1336.18. Teschke R, Glass X, Schulze J, Eickhoff A. Suspected Greater Celandine hepatotoxicity: liver-specific causality evaluation of published case reports from Europe. Eur J Gastroenterol Hepatol. 2012; 24:270–280.