Infect Chemother.
2010 Dec;42(6):362-368. 10.3947/ic.2010.42.6.362.
Current epidemiology and treatment of Clostridium difficile infection
- Affiliations
-
- 1Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea. paihj@hanyang.ac.kr
- KMID: 2170314
- DOI: http://doi.org/10.3947/ic.2010.42.6.362
Abstract
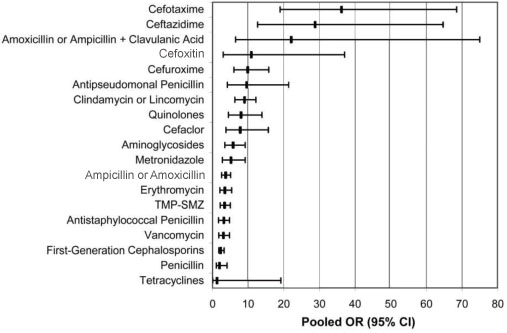
- With the emergence of B1/NAP1 strain of Clostridium difficile, epidemic feature of C. difficile infection (CDI) has been changing in North America. Clinical manifestation of CDI caused by B1/NAP1 strain is more severe resulting in high mortality and complications, and relapse rate is higher. In Korea, the epidemic feature of CDI is not clearly demonstrated yet. Infection by B1/NAP1 strain was reported recently, but the prevalence of the infections by those strains seems to be not high yet. For the management of CDI, conventional treatment of vancomycin or metronidazole is still the choice of treatment, however, several clinical trials of CDI treatment are progressing; fidaxomicin, monoclonal antibody, toxoid vaccine, and luminal non-toxigenic C. difficile infusion. Thus, it is expected to have more effective treatment or prevention modality of CDI in near future.
MeSH Terms
Figure
Cited by 3 articles
-
The Choice of Empirical Treatment of Uncomplicated Cystitis: No Longer Free Ride
Tae Hyong Kim
Infect Chemother. 2012;44(4):323-327. doi: 10.3947/ic.2012.44.4.323.The Choice of Empirical Treatment of Uncomplicated Cystitis: No Longer Free Ride
Tae Hyong Kim
Infect Chemother. 2012;44(4):323-327. doi: 10.3947/ic.2012.44.4.323.The clinical features and infectious etiologies of acute diarrhea in immunocompromised hosts
Jin Young Lee, Ye Na Kim, Namho Kim, Kyoung Soon Cho, Ji Young Park
Kosin Med J. 2017;32(2):191-203. doi: 10.7180/kmj.2017.32.2.191.
Reference
-
1. Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, Frost E, McDonald LC. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005. 366:1079–1084.
Article2. Clements AC, Magalhães RJ, Tatem AJ, Paterson DL, Riley TV. Clostridium difficile PCR ribotype 027: assessing the risks of further worldwide spread. Lancet Infect Dis. 2010. 10:395–404.
Article3. Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008. 46:Suppl 1. S12–S18.4. Kirkpatrick ID, Greenberg HM. Evaluating CT diagnosis of Clostridium difficile colitis: should CT guide therapy? AJR Am J Roentgenol. 2001. 176:635–639.5. Owens RC Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008. 46:Suppl 1. S19–S31.6. McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerg Infect Dis. 2006. 12:409–415.
Article7. Thibault A, Miller MA, Gaese C. Risk factors for the development of Clostridium difficile-associated diarrhea during a hospital outbreak. Infect Control Hosp Epidemiol. 1991. 12:345–348.
Article8. Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005. 294:2989–2995.
Article9. Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. New Engl J Med. 2000. 342:390–397.
Article10. Belmares J, Johnson S, Parada JP, Olson MM, Clabots CR, Bettin KM, Peterson LR, Gerding DN. Molecular epidemiology of Clostridium difficile over the course of 10 years in a tertiary care hospital. Clin Infect Dis. 2009. 49:1141–1147.
Article11. Muto CA, Pokrywka M, Shutt K, Mendelsohn AB, Nouri K, Posey K, Roberts T, Croyle K, Krystofiak S, Patel-Brown S, Pasculle AW, Paterson DL, Saul M, Harrison LH. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005. 26:273–280.
Article12. Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005. 173:1037–1042.13. Rouphael NG, O'Donnell JA, Bhatnagar J, Lewis F, Polgreen PM, Beekmann S, Guarner J, Killgore GE, Coffman B, Campbell J, Zaki SR, McDonald LC. Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008. 198:635.e1–635.e6.14. Kim J, Pai H, Seo MR, Oh SH, Kim GH, Kang JO, Choi TY. A Prospective Study For Clostridium difficile Associated Diarrhea In Korea. 2009. Philadelphia: IDSA;Abst 1023.15. Byun TJ, Han DS, Ahn SB, Cho HS, Kim TY, Eun CS, Jeon YC, Sohn JH, Kang JO. Clinical characteristics and changing epidemiology of Clostridium difficile-associated disease (CDAD). Korean J Gastroenterol. 2009. 54:13–19.
Article16. Kim H, Jeong SH, Roh KH, Hong SG, Kim JW, Shin MG, Kim MN, Shin HB, Uh Y, Lee H, Lee K. Investigation of toxin gene diversity. Molecular epidemiology, and antimicrobial resistance of Clostridium difficile isolated from 12 hospitals in South Korea. Korean J Lab Med. 2010. 30:491–497.
Article17. Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, Frost E, McDonald LC. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005. 366:1079–1084.
Article18. McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005. 353:2433–2441.
Article19. O'Connor D, Hynes P, Cormican M, Collins E, Corbett-Feeney G, Cassidy M. Evaluation of methods for detection of toxins in specimens of feces submitted for diagnosis of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2001. 39:2846–2849.20. Snell H, Ramos M, Longo S, John M, Hussain Z. Performance of the TechLab C. DIFF CHEK-60 enzyme immunoassay (EIA) in combination with the C. difficile Tox A/B II EIA kit, the Triage C. difficile panel immunoassay, and a cytotoxin assay for diagnosis of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2004. 42:4863–4865.
Article21. Ticehurst JR, Aird DZ, Dam LM, Borek AP, Hargrove JT, Carroll KC. Effective detection of toxigenic Clostridium difficile by a two-step algorithm including tests for antigen and cytotoxin. J Clin Microbiol. 2006. 44:1145–1149.
Article22. Knetsch CW, Bakker D, de Boer RF, Sanders I, Hofs S, Kooistra-Smid AM, Corver J, Eastwood K, Wilcox MH, Kuijper EJ. Comparison of real-time PCR techniques to cytotoxigenic culture methods for diagnosing Clostridium difficile infection. J Clin Microbiol. 2010 Oct 27. [Epub ahead of print].23. Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. Society for Healthcare Epidemiology of America. Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010. 31:431–455.
Article24. Teasley DG, Gerding DN, Olson MM, Peterson LR, Gebhard RL, Schwartz MJ, Lee JT Jr. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983. 2:1043–1046.25. Musher DM, Aslam S, Logan N, Nallacheru S, Bhaila I, Borchert F, Hamill RJ. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005. 40:1586–1590.
Article26. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007. 45:302–307.
Article27. Louie TJ, Gerson M, Grimard D, Johnson S, Poirier A, Weiss K, et al. Results of a phase III trial comparing tolevamer, vancomycin and metronidazole in patients with Clostridium difficile-associated diarrhea (CDAD). Program and abstracts of the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy. 2007. Chicago: ICAAC;Abstract K-425a.28. Pepin J, Alary ME, Valiquette L, Raiche E, Ruel J, Fulop K, Godin D, Bourassa C. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005. 40:1591–1597.
Article29. Wenisch C, Parschalk B, Hasenhüdl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996. 22:813–818.
Article30. Musher DM, Logan N, Bressler AM, Johnson DP, Rossignol JF. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clin Infect Dis. 2009. 48:e41–e46.31. Lagrotteria D, Holmes S, Smieja M, Smaill F, Lee C. Prospective, randomized inpatient study of oral metronidazole versus oral metronidazole and rifampin for treatment of primary episode of Clostridium difficile-associated diarrhea. Clin Infect Dis. 2006. 43:547–552.
Article32. Johnson S, Schriever C, Patel U, Patel T, Hecht DW, Gerding DN. Rifaximin Redux: treatment of recurrent Clostridium difficile infections with rifaximin immediately post-vancomycin treatment. Anaerobe. 2009. 15:290–291.
Article33. Louie T, Mullane K, Weiss K, et al. A randomized, double-blind clinical trial of OPT-80 versus vancomycin in Clostridium difficile infection. Program and abstracts of the 19th European Congress of Clinical Microbiology and Infectious Diseases. 2009. Helsinki: ECCMID;Abstract 0148.34. Louie TJ, Emery J, Krulicki W, Byrne B, Mah M. OPT-80 eliminates Clostridium difficile and is sparing of bacteroides species during treatment of C. difficile infection. Antimicrob Agents Chemother. 2009. 53:261–263.
Article35. Barker RH Jr, Dagher R, Davidson DM, Marquis JK. Review article: tolevamer, a novel toxin-binding polymer: overview of preclinical pharmacology and physiochemical properties. Aliment Pharmacol Ther. 2006. 24:1525–1534.
Article36. Louie TJ, Peppe J, Watt CK, Johnson D, Mohammed R, Dow G, Weiss K, Simon S, John JF Jr, Garber G, Chasan-Taber S, Davidson DM. Tolevamer Study Investigator Group. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis. 2006. 43:411–420.
Article37. Bouza E, Dryden M, Mohammed R, Peppe J, Chasan-Taber S, Donovan J, et al. Results of a phase III trial comparing tolevamer, vancomycin and metronidazole in patients with Clostridium difficile-associated diarrhoea. 2008. In : The 18th European congress of clinical microbiology and infectious diseases; Barcelona: ECCMID;Abstract 0464.38. Baines SD, Freeman J, Wilcox MH. Tolevamer is not efficacious in the neutralization of cytotoxin in a human gut model of Clostridium difficile infection. Antimicrob Agents Chemother. 2009. 53:2202–2204.
Article39. McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006. 101:812–822.
Article40. Pillai A, Nelson R. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008. CD004611.41. Lowy I, Molrine DC, Leav BA, Blair BM, Baxter R, Gerding DN, Nichol G, Thomas WD Jr, Leney M, Sloan S, Hay CA, Ambrosino DM. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010. 362:197–205.
Article42. Gerding DN, Johnson S. Management of Clostridium difficile infection: thinking inside and outside the box. Clin Infect Dis. 2010. 51:1306–1313.
Article43. Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe. 2009. 15:285–289.
Article44. Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010. 44:354–360.
Article