Endocrinol Metab.
2010 Sep;25(3):213-216. 10.3803/EnM.2010.25.3.213.
Sustained Maintenance of Normal Insulin-like Growth Factor-I during Pregnancy and Successful Delivery in an Acromegalic Patient with Octreotide-LAR(R) Treatment
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. kw1234@skku.edu
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Gyeongsang National University School of Medicine, Jinju, Korea.
- KMID: 2169059
- DOI: http://doi.org/10.3803/EnM.2010.25.3.213
Abstract
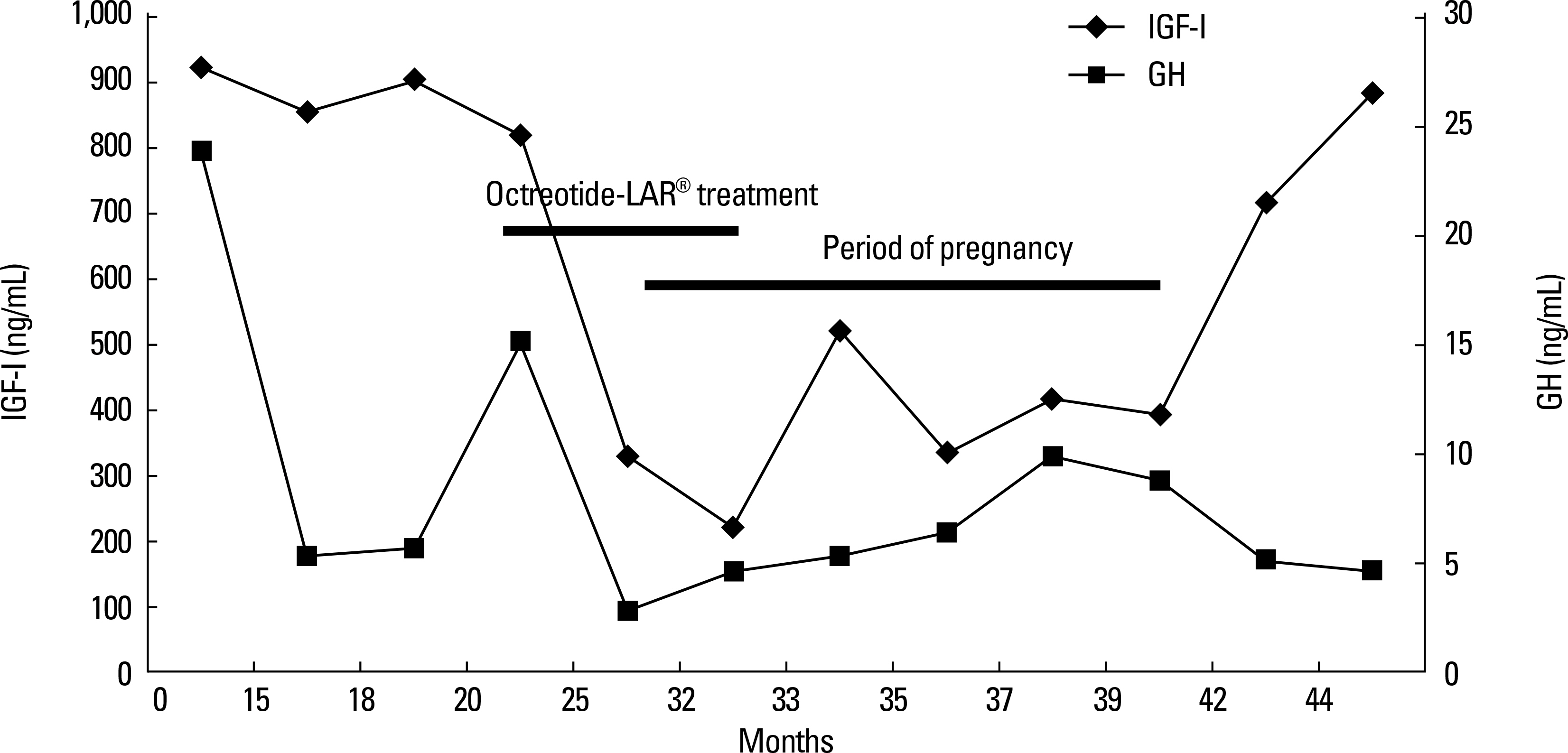
- We report here on a 34-year-old Korean woman with active acromegaly and who received Octreotide-LAR(R) for 12 months following transsphenoidal pituitary surgery. During Octreotide-LAR(R) treatment, the clinical improvement was paralleled with the decrease of the growth hormone levels to 1.1 ng/mL and the insulin-like growth factor-I (IGF-I) levels to 345.5 ng/mL. Octreotide-LAR(R) was discontinued when the patient was found to be at the 12th week of pregnancy. During pregnancy, the patient experienced clinical well-being and she maintained her IGF-I levels within the normal range for her age-matched despite discontinuation of Octreotide-LAR(R) treatment at early gestation. She delivered a full-term healthy male infant. The serum IGF-I levels of the patient increased progressively increased after delivery. This report describes a successful pregnancy in an acromegalic woman who was exposed to Octreotide-LAR(R) during the early gestational period. She and who showed an unexpected pattern of persistently normal IGF-I levels through the pregnancy despite discontinuation of Octreotide-LAR(R) therapy.
MeSH Terms
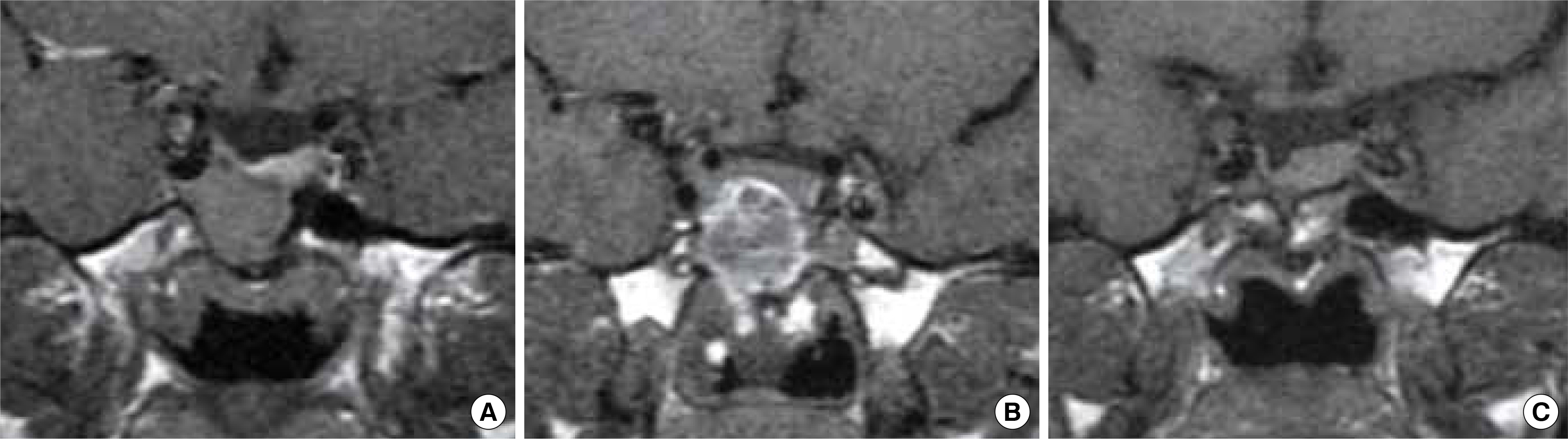
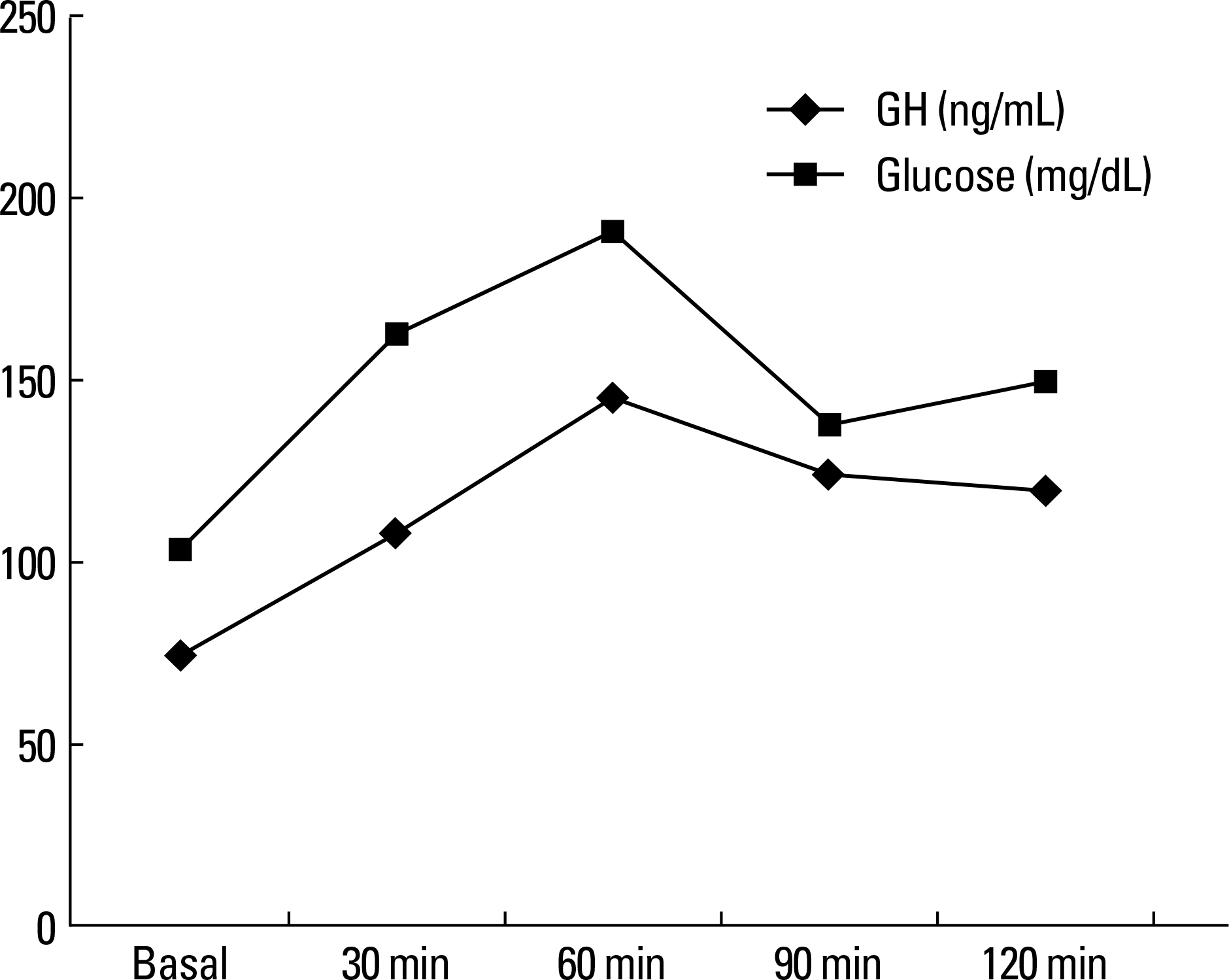
Figure
Reference
-
References
1. Takano T, Saito J, Soyama A, Ito H, Iizuka T, Yoshida T, Nishikawa T. Normal delivery following an uneventful pregnancy in a Japanese acromegalic patient after discontinuation of octreotide long acting release formulation at an early phase of pregnancy. Endocr J. 53:209–212. 2006.
Article2. Fassnacht M, Capeller B, Arlt W, Steck T, Allolio B. Octreotide LAR treatment throughout pregnancy in an acromegalic woman. Clin Endocrinol (Oxf). 55:411–415. 2001.
Article3. Takeuchi K, Funakoshi T, Oomori S, Maruo T. Successful pregnancy in an acromegalic woman treated with octreotide. Obstet Gynecol. 93:848. 1999.
Article4. Serri O, Lanoie G. Successful pregnancy in a woman with acromegaly treated with Octreotide Long-acting Release. Endocrinologist. 13:17–19. 2003.
Article5. Hierl T, Ziegler R, Kasperk C. Pregnancy in persistent acromegaly. Clin Endocrinol (Oxf). 53:262–263. 2000.
Article6. Goluboff LG, Ezrin C. Effect of pregnancy on the somatotroph and the prolactin cell of the human adenohypophysis. J Clin Endocrinol Metab. 29:1533–1538. 1969.
Article7. Molitch ME. Pregnancy and the hyperprolactinemic woman. N Engl J Med. 312:1364–1370. 1985.
Article8. Demura R, Ono M, Demura H, Shizume K, Oouchi H. Prolactin directly inhibits basal as well as gonadotropin-stimulated secretion of progesterone and 17 beta-estradiol in the human ovary. J Clin Endocrinol Metab. 54:1246–1250. 1982.9. Choi H, Lee Y, Chung IH, Koh JH, Kim MJ, Shin YG, Chung CH. A case of two consecutive deliveries in a woman with acromegaly. Korean J Med. 67:662–666. 2004.10. Wilson DM, Bennett A, Adamson GD, Nagashima RJ, Liu F, DeNatale ML, Hintz RL, Rosenfeld RG. Somatomedins in pregnancy: a cross-sectional study of insulinlike growth factors I and II and somatomedin peptide content in normal human pregnancies. J Clin Endocrinol Metab. 55:858–861. 1982.
Article11. Beckers A, Stevenaert A, Foidart JM, Hennen G, Frankenne F. Placental and pituitary growth hormone secretion during pregnancy in acromegalic women. J Clin Endocrinol Metab. 71:725–731. 1990.
Article12. Herman-Bonert V, Seliverstov M, Melmed S. Pregnancy in acromegaly: successful therapeutic outcome. J Clin Endocrinol Metab. 83:727–731. 1998.
Article13. Okada Y, Morimoto I, Ejima K, Yoshida K, Kashimura M, Fujihira T, Eto S. A case of active acromegalic woman with a marked increase in serum insulinlike growth factor-I levels after delivery. Endocr J. 44:117–120. 1997.14. Clemmons DR, Underwood LE, Ridgway EC, Kliman B, Kjellberg RN, Van Wyk JJ. Estradiol treatment of acromegaly. Reduction of immunoreactive somatomedin-C and improvement in metabolic status. Am J Med. 69:571–575. 1980.
Article15. Parkinson C, Ryder WD, Trainer PJ. Sensus Acromegaly Study Group. The relationship between serum GH and serum IGF-I in acromegaly is gender-specific. J Clin Endocrinol Metab. 86:5240–5244. 2001.
Article16. Lee KW, Nam MS. Medical therapy of somato statin analog in acromegaly. J Korean Soc Endocrinol. 17:629–634. 2002.17. Mikhail N. Octreotide treatment of acromegaly during pregnancy. Mayo Clin Proc. 77:297–298. 2002.
Article18. de Menis E, Billeci D, Marton E, Gussoni G. Uneventful pregnancy in an acromegalic patient treated with slow-release lanreotide: a case report. J Clin Endocrinol Metab. 84:1489. 1999.
Article19. Caron P, Gerbeau C, Pradayrol L. Maternal-fetal transfer of octreotide. N Engl J Med. 333:601–602. 1995.
Article20. Caron P, Gerbeau C, Pradayrol L, Simonetta C, Bayard F. Successful pregnancy in an infertile woman with a thyrotropin-secreting macroadenoma treated with somatostatin analog (octreotide). J Clin Endocrinol Metab. 81:1164–1168. 1996.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Efficacy of Octreotide Long-acting Repeatable and Lanreotide Autogel in Acromegalic Patients
- Acromegaly with Diabetes Insipidus after Pituitary Tumor Removal: Successful Pregnancy and Delivery
- Efficacy of Octreotide LAR in Acromegalic Patients
- Acromegaly improved with sandostatin LAR treatment
- The Effect of Octreotide LAR on GH and TSH Co-Secreting Pituitary Adenoma