J Korean Endocr Soc.
2010 Mar;25(1):37-45. 10.3803/jkes.2010.25.1.37.
Comparison of the Efficacy of Octreotide Long-acting Repeatable and Lanreotide Autogel in Acromegalic Patients
- Affiliations
-
- 1Department of Internal Medicine, Chungnam National University, College of Medicine, Korea.
- 2Department of Internal Medicine, Dankook University, College of Medicine, Korea.
- KMID: 2177859
- DOI: http://doi.org/10.3803/jkes.2010.25.1.37
Abstract
- BACKGROUND
Somatostatin analogues have been used as the first-line medical therapy for active acromegaly that is not completely cured, or which recurs after surgery. The aim of this study was to compare the effects of octreotide long-acting repeatable (LAR) and lanreotide Autogel. Such a comparison has not been reported in Korea.
METHODS
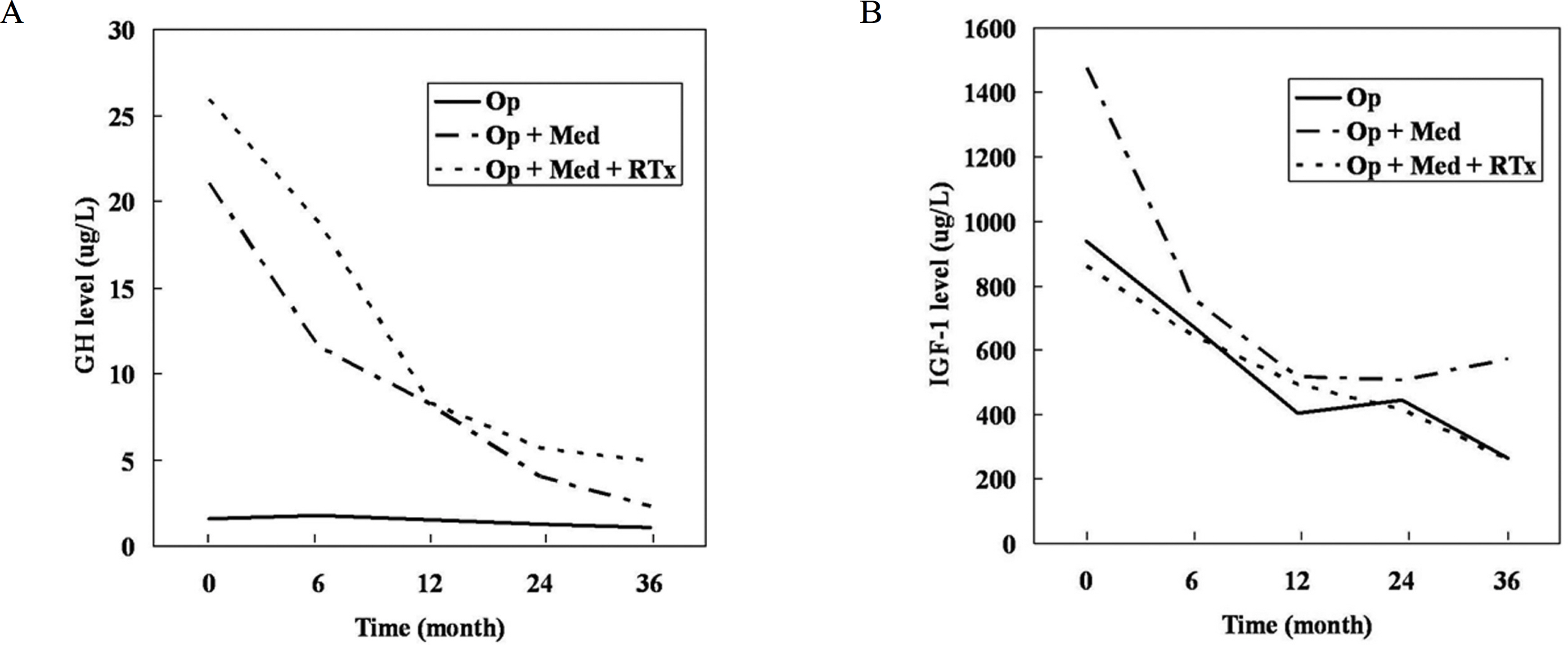
Twenty-seven patients who had previously undergone surgery for acromegaly from December 2003 to March 2005 were included. We retrospectively investigated eight patients who underwent operation only and 19 patients who additionally received medical treatment after surgery (octreotide LAR, n = 5; lanreotide Autogel, n = 5). Growth hormone (GH) and insulin-like growth factor-I (IGF-I) levels were measured.
RESULTS
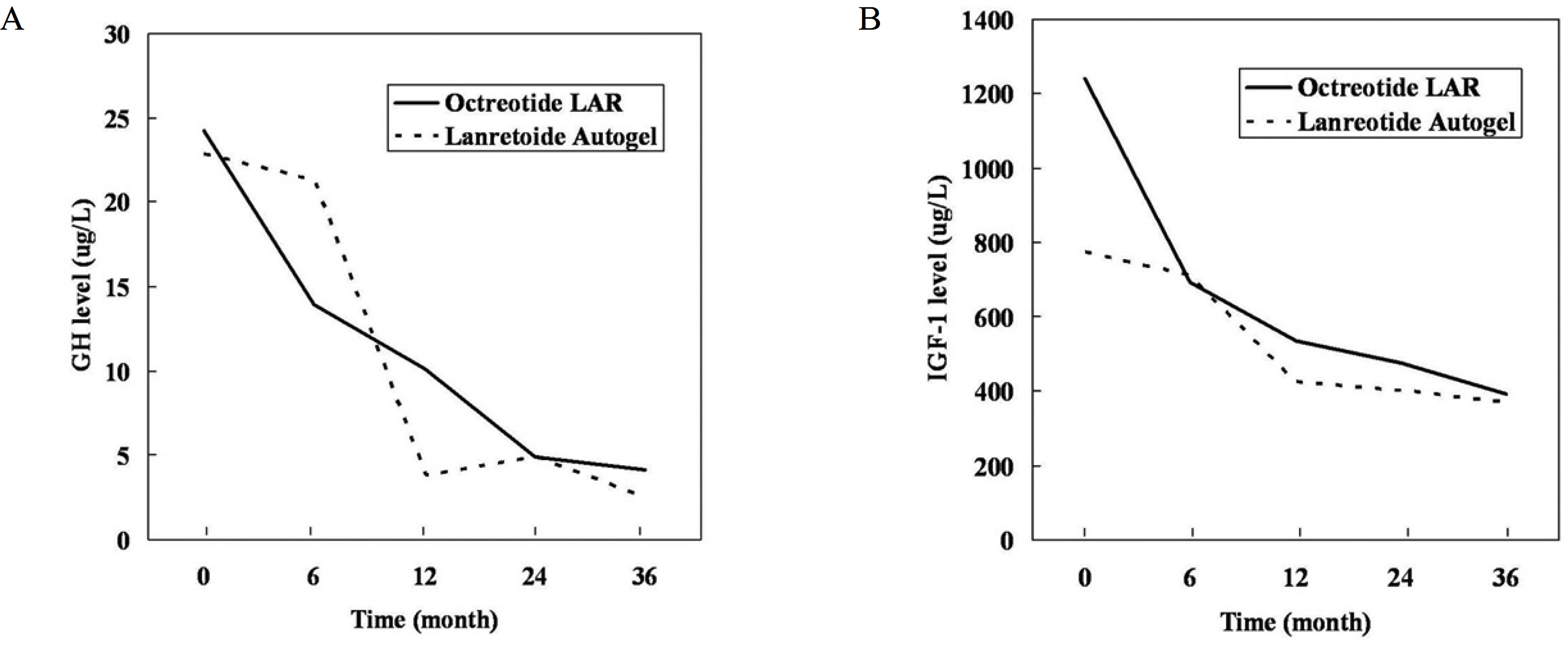
The mean pre-operative and post-operative levels of GH were lower in patients who underwent surgery only than in those who received adjuvant therapy, but IGF-I levels were not significantly different. In the 19 patients receiving medical treatment after unsuccessful surgery, the mean baseline GH levels were 24.2 microgram/L for octreotide LAR and 22.8 microgram/L for lanreotide Autogel (P = 0.711), and the mean GH levels 36 months post-treatment were 4.1 microgram/L and 2.5 microgram/L, respectively (P = 0.794). GH < 2.5 microgram/L represented 30% of octreotide LAR patients and 33.3% of lanreotide Autogel patients (P = 0.91). Patients with normal IGF-I levels represented 54.5% and 66.7%, respectively (P = 0.71).
CONCLUSION
No significant difference in therapeutic effect of octreotide LAR and lanreotide Autogel was evident in 19 Korean acromegalic patients who were not completely cured by surgery and radiation therapy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Medical Treatment with Somatostatin Analogues in Acromegaly: Position Statement
Sang Ouk Chin, Cheol Ryong Ku, Byung Joon Kim, Sung-Woon Kim, Kyeong Hye Park, Kee Ho Song, Seungjoon Oh, Hyun Koo Yoon, Eun Jig Lee, Jung Min Lee, Jung Soo Lim, Jung Hee Kim, Kwang Joon Kim, Heung Yong Jin, Dae Jung Kim, Kyung Ae Lee, Seong-Su Moon, Dong Jun Lim, Dong Yeob Shin, Se Hwa Kim, Min Jeong Kwon, Ha Young Kim, Jin Hwa Kim, Dong Sun Kim, Chong Hwa Kim
Endocrinol Metab. 2019;34(1):53-62. doi: 10.3803/EnM.2019.34.1.53.
Reference
-
References
1. Holdaway IM, Rajasoorya RC, Gamble GD. Factors influencing mortality in acromegaly. J Clin Endocrinol Metab. 89:667–674. 2004.
Article2. Rajasoorya C, Holdaway IM, Wrightson P, Scott DJ, Ibbertson HK. Determinants of clinical outcome and survival in acromegaly. Clin Endocrinol (Oxf). 41:95–102. 1994.
Article3. Colao A, Pivonello R, Auriemma RS, Briganti F, Galdiero M, Tortora F, Caranci F, Cirillo S, Lombardi G. Predictors of tumor shrinkage after primary therapy with somatostatin analogs in acromegaly: Predictors of tumor shrinkage after primary therapy with somatostatin analogs in acromegaly: a prospective study in 99 patients. J Clin Endocrinol Metab. 91:2112–2118. 2006.4. Powell JS, Wardlaw SL, Post KD, Freda PU. Outcome of radiotherapy for acromegaly using normalization of insulinlike growth factor I to define cure. J Clin Endocrinol Metab. 85:2068–2071. 2000.
Article5. Melmed S. Medical progress: Acromegaly. N Engl J Med. 355:2558–2573. 2006.6. Freda PU, Katznelson L, van der Lely AJ, Reyes CM, Zhao S, Rabinowitz D. Long-acting somatostatin analog therapy of acromegaly: a metaanalysis. J Clin Endocrinol Metab. 90:4465–4473. 2005.
Article7. Chanson P, Borson-Chazot F, Kuhn JM, Blumberg J, Maisonobe P, Delemer B. Lanreotide Acromegaly Study Group. Control of IGF-I levels with titrated dosing of lanreotide Autogel over 48 weeks in patients with acromegaly. Clin Endocrinol (Oxf). 69:299–305. 2008.8. Bonadonna S, Mazziotti G, Nuzzo M, Bianchi A, Fusco A, De Marinis L, Giustina A. Increased prevalence of radiological spinal deformities in active acromegaly: a cross-sectional study in postmenopausal women. J Bone Miner Res. 20:1837–1844. 2005.
Article9. Rosenow F, Reuter S, Deuss U, Szelies B, Hilgers RD, Winkelmann W, Heiss WD. Sleep apnoea in treated acromegaly: relative frequency and predisposing factors. Clin Endocrinol (Oxf). 45:563–569. 1996.
Article10. Orme SM, McNally RJ, Cartwright RA, Belchetz PE. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab. 83:2730–2734. 1998.11. Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, Veldhuis J, Wass J, Von Werder K, Melmed S. Criteria for cure of acromegaly: Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab. 85:526–529. 2000.12. Biermasz NR, Pereira AM, Smit JW, Romijn JA, Roelfsema F. Morbidity after longterm remission for acromegaly: persisting joint-related complaints cause reduced quality of life. J Clin Endocrinol Metab. 90:2731–2739. 2005.
Article13. Cappabianca P, Cavallo LM, de Divitiis E. Endoscopic endonasal transsphenoidal surgery. Neurosurgery. 55:933–940. 2004.
Article14. Barker FG 2nd, Klibanski A, Swearingen B. Transsphenoidal surgery for pituitary tumors in the United States, 1996–2000: mortality, morbidity, and the effects of hospital and surgeon volume. J Clin Endocrinol Metab. 88:4709–4719. 2003.
Article15. Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical ‘cure'. Eur J Endocrinol. 152:379–387. 2005.
Article16. Barrande G, Pittino-Lungo M, Coste J, Ponvert D, Bertagna X, Luton JP, Bertherat J. Hormonal and metabolic effects of radiotherapy in acromegaly: longterm results in 128 patients followed in a single center. J Clin Endocrinol Metab. 85:3779–3785. 2000.
Article17. Brada M, Burchell L, Ashley S, Traish D. The incidence of cerebrovascular accidents in patients with pituitary adenoma. Int J Radiat Oncol Biol Phys. 45:693–698. 1999.
Article18. Bush ZM, Vance ML. Management of acromegaly: is there a role for primary medical therapy? Rev Endocr Metab Disord. 9:83–94. 2008.
Article19. Murray RD, Melmed S. A critical analysis of clinically available somatostatin analog formulations for therapy of acromegaly. J Clin Endocrinol Metab. 93:2957–2968. 2008.
Article20. Kim JY, Jee JH, Yoon CH, Chung YJ, Lee BW, Cho GY, Kim SY, Chung JH, Min YK, Lee MS, Lee MK, Kim KW. Efficacy of octreotide LAR in acromegalic patients. J Korean Soc Endocrinol. 20:344–352. 2005.
Article21. Yang SJ, Seo YJ, Eun CR, Chung HS, Choi HJ, Kim NH, Kim HS, Park JR, Kim DJ, Yoo HJ, Park SY, Lee YJ, Ryu OH, Kim HY, Lee KW, Seo JA, Kim SG, Choi DS, Baik SH. Therapeutic effects of a long-acting formulation of lanreotide in Korean patients with acromegaly. Korean J Med. 73:50–57. 2007.22. Jallad RS, Musolino NR, Salgado LR, Bronstein MD. Treatment of acromegaly with octreotide-LAR: extensive experience in a Brazilian institution. Clin Endocrinol (Oxf). 63:168–175. 2005.
Article23. Mercado M, Borges F, Bouterfa H, Chang TC, Chervin A, Farrall AJ, Patocs A, Petersenn S, Podoba J, Safari M, Wardlaw J. SMS995B2401 Study Group. A prospective, multicentre study to investigate the efficacy, safety and tolerability of octreotide LAR (long-acting repeatable octreotide) in the primary therapy of patients with acromegaly. Clin Endocrinol (Oxf). 66:859–868. 2007.24. Andries M, Glintborg D, Kvistborg A, Hagen C, Andersen M. A 12-month randomized crossover study on the effects of lanreotide Autogel and octreotide long-acting repeatable on GH and IGF-I in patients with acromegaly. Clin Endocrinol (Oxf). 68:473–480. 2008.25. Ashwell SG, Bevan JS, Edwards OM, Harris MM, Holmes C, Middleton MA, James RA. The efficacy and safety of lanreotide Autogel in patients with acromegaly previously treated with octreotide LAR. Eur J Endocrinol. 150:473–480. 2004.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Efficacy of Octreotide Long-acting Repeatable and Lanreotide Autogel in Acromegalic Patients
- Letter: Comparison of the Efficacy of Octreotide Long-acting Repeatable and Lanreotide Autogel in Acromegalic Patients (J Korean Endocr Soc 25:37-45, 2010)
- Response: Comparison of the Efficacy of Octreotide Long-acting Repeatable and Lanreotide Autogel in Acromegalic Patients (J Korean Endocr Soc 25:37-45, 2010)
- Therapeutic effects of a long-acting formulation of lanreotide in Korean patients with acromegaly
- SR (Slow-Replase) Lanreotide Treatment in Acromegalic Patients