J Bacteriol Virol.
2012 Dec;42(4):263-275. 10.4167/jbv.2012.42.4.263.
Application of Metagenomic Techniques: Understanding the Unrevealed Human Microbiota and Explaining the in Clinical Infectious Diseases
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Chung-Ang University, Seoul, Korea. kimwy@cau.ac.kr
- KMID: 2168661
- DOI: http://doi.org/10.4167/jbv.2012.42.4.263
Abstract
- Uncultured microorganisms comprise the majority of the planet's biological diversity. In many environments, as many as 99% of the microorganisms cannot be cultured by standard techniques, and the uncultured fraction includes diverse organisms that are only distantly related to the cultured ones. Therefore, culture-independent methods are essential to understand the genetic diversity, population structure, and ecological roles of the majority of microorganisms. Recently, new techniques for studying microbial communities, collectively called metagenomics, have been developed to overcome the limitations of culturing. This review assesses the potential of metagenomic techniques to analyze the relative abundance of microbial species under varying human environmental conditions and to discover infectious causes of unexplained human diseases.
Figure
Cited by 2 articles
-
Roles of Enteric Microbial Composition and Metabolism in Health and Diseases
Jung Mogg Kim
Korean J Gastroenterol. 2013;62(4):191-205. doi: 10.4166/kjg.2013.62.4.191.Molecular Methods for Studying the Human Microbiota
Yoon Hee Choi, Jin Chung, Hee Sam Na
J Bacteriol Virol. 2013;43(1):67-72. doi: 10.4167/jbv.2013.43.1.67.
Reference
-
1. Staley JT, Konopka A. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu Rev Microbiol. 1985. 39:321.
Article2. Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995. 59:143–169.
Article3. Hugenholtz P, Goebel BM, Pace NR. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol. 1998. 180:4765–4774.
Article4. Hugenholtz P, Hooper SD, Kyrpides NC. Focus: Synergistetes. Environ Microbiol. 2009. 11:1327–1329.5. Auguet JC, Barberan A, Casamayor EO. Global ecological patterns in uncultured Archaea. ISME J. 2010. 4:182–190.
Article6. Giovannoni SJ, Britschgi TB, Moyer CL, Field KG. Genetic diversity in Sargasso Sea bacterioplankton. Nature. 1990. 345:60–63.
Article7. Pace NR. A molecular view of microbial diversity and the biosphere. Science. 1997. 276:734–740.
Article8. Rappé MS, Giovannoni SJ. The uncultured microbial majority. Annu Rev Microbiol. 2003. 57:369–394.
Article9. Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology. 2004. 150:2565–2573.
Article10. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005. 43:5721–5732.
Article11. Bogaert D, Keijser B, Huse S, Rossen J, Veenhoven R, van Gils E, et al. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS One. 2011. 6:e17035.
Article12. Greninger AL, Chen EC, Sittler T, Scheinerman A, Roubinian N, Yu G, et al. A metagenomic analysis of pandemic influenza A (2009 H1N1) infection in patients from North America. PLoS One. 2010. 5:e13381.
Article13. Finkbeiner SR, Allred AF, Tarr PI, Klein EJ, Kirkwood CD, Wang D. Metagenomic analysis of human diarrhea: viral detection and discovery. PLoS Pathog. 2008. 4:e1000011.
Article14. Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006. 312:1355–1359.
Article15. Bae JW. Recent Methodological Approaches to Human Microbiome. J Bacteriol Virol. 2011. 41:1–7.
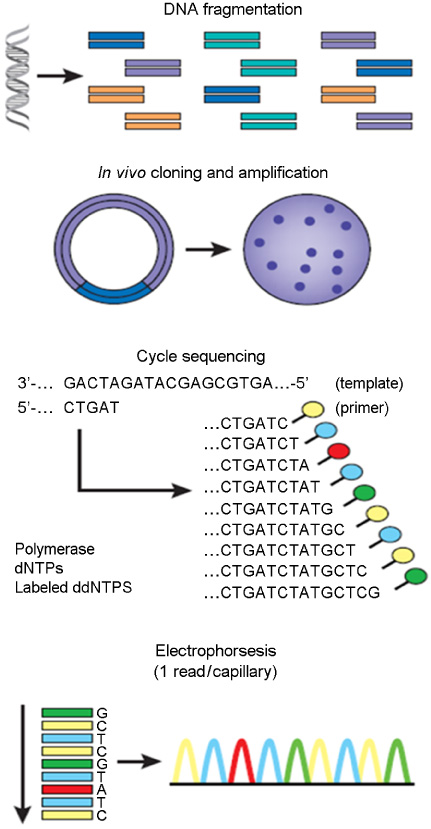
Article16. Sanger F, Air GM, Barrell BG, Brown NL, Coulson AR, Fiddes CA, et al. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977. 265:687–695.17. Swerdlow H, Wu SL, Harke H, Dovichi NJ. Capillary gel electrophoresis for DNA sequencing. Laser-induced fluorescence detection with the sheath flow cuvette. J Chromatogr. 1990. 516:61–67.18. Hunkapiller T, Kaiser RJ, Koop BF, Hood L. Large-scale and automated DNA sequence determination. Science. 1991. 254:59–67.
Article19. Noguchi H, Park J, Takagi T. MetaGene: prokaryotic gene finding from environmental genome shotgun sequences. Nucleic Acids Res. 2006. 34:5623–5630.
Article20. Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998. 8:175–185.
Article21. Keith CS, Hoang DO, Barrett BM, Feigelman B, Nelson MC, Thai H, et al. Partial sequence analysis of 130 randomly selected maize cDNA clones. Plant Physiol. 1993. 101:329–332.
Article22. Richter DC, Ott F, Auch AF, Schmid R, Huson DH. MetaSim: a sequencing simulator for genomics and metagenomics. PLoS One. 2008. 3:e3373.
Article23. Ronaghi M, Uhlén M, Nyrén P. A sequencing method based on real-time pyrophosphate. Science. 1998. 281:363. 365.
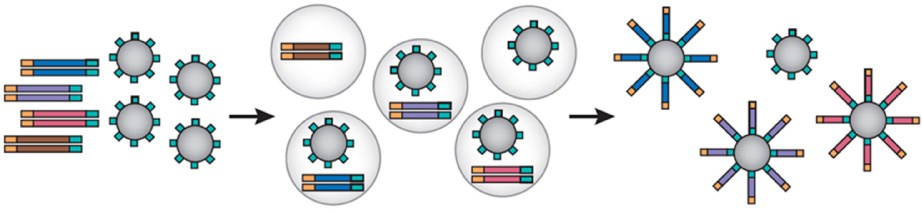
Article24. Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005. 437:376–380.25. Huse SM, Huber JA, Morrison HG, Sogin ML, Welch DM. Accuracy and quality of massively parallel DNA pyrosequencing. Genome Biol. 2007. 8:R143.
Article26. Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008. 26:1135–1145.
Article27. Rusk N, Kiermer V. Primer: Sequencing--the next generation. Nat Methods. 2008. 5:15.
Article28. Schuster SC. Next-generation sequencing transforms today\'s biology. Nat Methods. 2008. 5:16–18.
Article29. Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977. 74:5463–5467.
Article30. Dressman D, Yan H, Traverso G, Kinzler KW, Vogelstein B. Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations. Proc Natl Acad Sci U S A. 2003. 100:8817–8822.
Article31. Hall N. Advanced sequencing technologies and their wider impact in microbiology. J Exp Biol. 2007. 210:1518–1525.
Article32. Pettersson E, Lundeberg J, Ahmadian A. Generations of sequencing technologies. Genomics. 2009. 93:105–111.
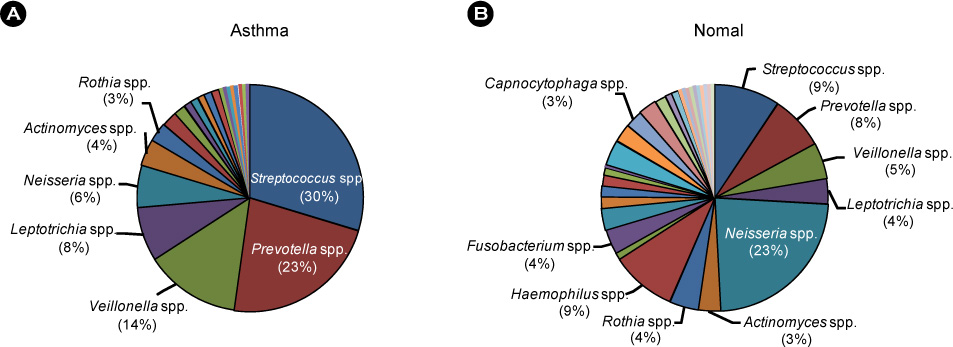
Article33. Ramsey CD, Gold DR, Litonjua AA, Sredl DL, Ryan L, Celedón JC. Respiratory illnesses in early life and asthma and atopy in childhood. J Allergy Clin Immunol. 2007. 119:150–156.
Article34. Huffnagle GB. The microbiota and allergies/asthma. PLoS Pathog. 2010. 6:e1000549.
Article35. Noverr MC, Huffnagle GB. The 'microflora hypothesis' of allergic diseases. Clin Exp Allergy. 2005. 35:1511–1520.
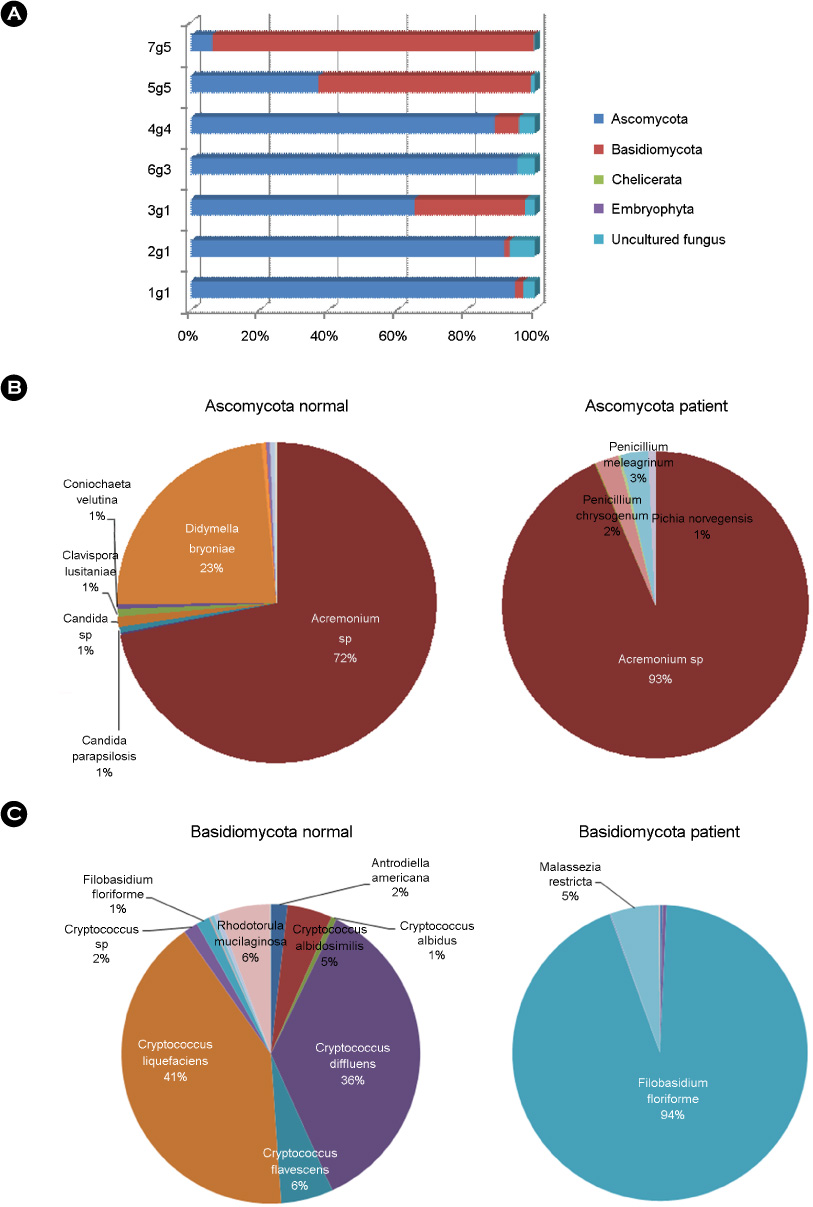
Article36. Park HK, Ha MH, Park SG, Kim MN, Kim BJ, Kim W. Characterization of the fungal microbiota (mycobiome) in healthy and dandruff-afflicted human scalps. PLoS One. 2012. 7:e32847.
Article37. Piérard-Franchimont C, Xhauflaire-Uhoda E, Piérard GE. Revisiting dandruff. Int J Cosmet Sci. 2006. 28:311–318.
Article38. Ranganathan S, Mukhopadhyay T. Dandruff: the most commercially exploited skin disease. Indian J Dermatol. 2010. 55:130–134.
Article39. Jo JH, Jang HS, Ko HC, Kim MB, Oh CK, Kwon YW, et al. Pustular psoriasis and the Kobner phenomenon caused by allergic contact dermatitis from zinc pyrithione-containing shampoo. Contact Dermatitis. 2005. 52:142–144.
Article40. Piérard GE, Piérard-Franchimont C. Squamometry in acute photodamage. Skin Res Technol. 1995. 1:137–139.
Article41. Gupta AK, Madzia SE, Batra R. Etiology and Management of Seborrheic Dermatitis. Dermatology. 2004. 208:89–93.
Article42. Leyden JJ, McGinley KJ, Kligman AM. Role of microorganisms in dandruff. Arch Dermatol. 1976. 112:333–338.
Article43. McGinley KJ, Leyden JJ, Marples RR, Kligman AM. Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrheic dermatitis. J Invest Dermatol. 1975. 64:401–405.
Article44. Guého E, Boekhout T, Ashbee HR, Guillot J, Van Belkum A, Faergemann J. The role of Malassezia species in the ecology of human skin and as pathogens. Med Mycol. 1998. 36:220–229.45. Sugita T, Takashima M, Shinoda T, Suto H, Unno T, Tsuboi R, et al. New yeast species, Malassezia dermatis, isolated from patients with atopic dermatitis. J Clin Microbiol. 2002. 40:1363–1367.
Article46. Erchiga VC, Florencio VD. Malassezia species in skin diseases. Curr Opin Infect Dis. 2002. 15:133–142.47. Gupta AK, Kohli Y, Faergemann J, Summerbell RC. Epidemiology of Malassezia yeasts associated with pityriasis versicolor in Ontario, Canada. Med Mycol. 2001. 39:199–206.
Article48. Prohić A. Identification of Malassezia species isolated from scalp skin of patients with psoriasis and healthy subjects. Acta Dermatovenerol Croat. 2003. 11:10–16.49. Sugita T, Suto H, Unno T, Tsuboi R, Ogawa H, Shinoda T, et al. Molecular analysis of Malassezia microflora on the skin of atopic dermatitis patients and healthy subjects. J Clin Microbiol. 2001. 39:3486–3490.
Article50. Kirk PM, Cannon PF, Minter DW, JA S, editors. Dictionary of the Fungi. 2008. 10th ed. Wallingford: CABI.51. Pan W, Liao W, Hagen F, Theelen B, Shi W, Meis JF, et al. Meningitis caused by Filobasidium uniguttulatum: case report and overview of the literature. Mycoses. 2012. 55:105–109.52. Kwon-Chung KJ. Perfect state of Cryptococcus uniguttulatus. Int J Syst Bacteriol. 1977. 27:293–299.53. Kwon-Chung KJ, Boekhout T, Wickes B, Fell J. Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A, editors. Systematics of the Genus Cryptococcus and its type species C. neoformans. Cryptococcus: From human pathogen to model yeast. 2011. Washington, DC: ASM press;3–16.54. Fell JW, Boekhout T, Fonseca A, Scorzetti G, Statzell-Tallman A. Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int J Syst Evol Microbiol. 2000. 50:1351–1371.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Status and Future Promise of the Human Microbiome
- Human Immunodeficiency Virus Infection and Gut Microbiota: A Review on Gut Microbiome Associated with Immune Activation and Metabolic Diseases
- Microbiota Influences Vaccine and Mucosal Adjuvant Efficacy
- Intestinal Microbiota Contributes to Energy Balance, Metabolic Inflammation, and Insulin Resistance in Obesity
- Fecal Microbiota Transplantation and the Brain Microbiota in Neurological Diseases