ABO Incompatability in Liver Transplantation
- Affiliations
-
- 1Department of Surgery, Division of Liver Transplantation and Hepatobiliary Surgry, Asan Medical Center, Ulsan University College of Medicine, Seoul, Korea. drsong71@amc.seoul.kr
- KMID: 2168353
- DOI: http://doi.org/10.7599/hmr.2014.34.4.202
Abstract
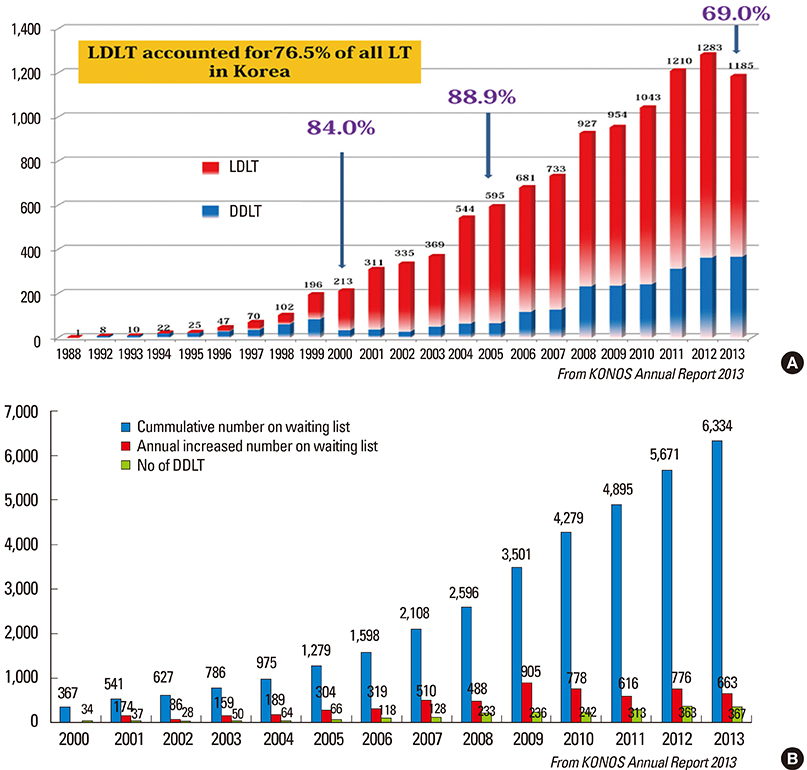
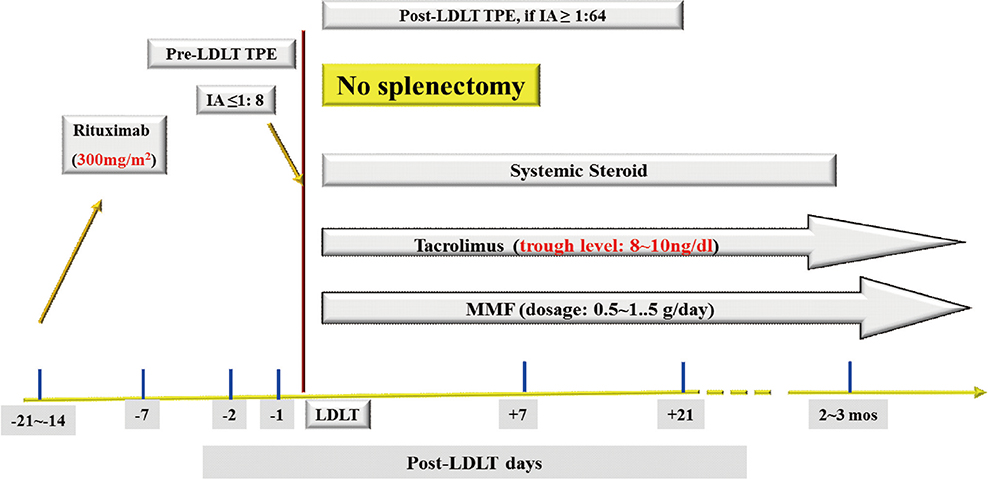
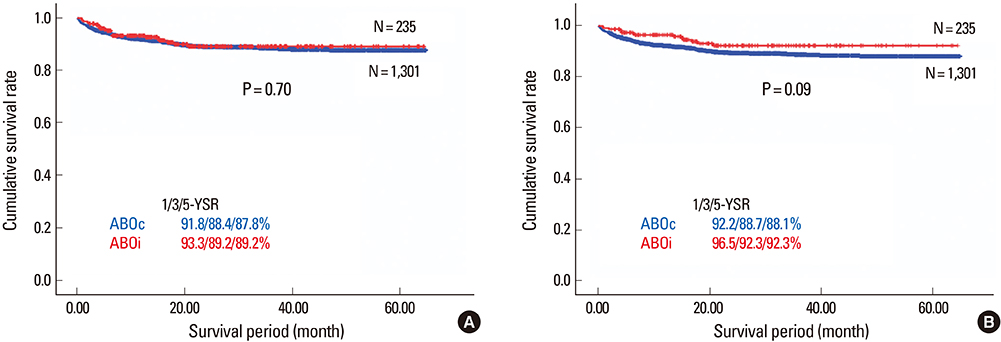
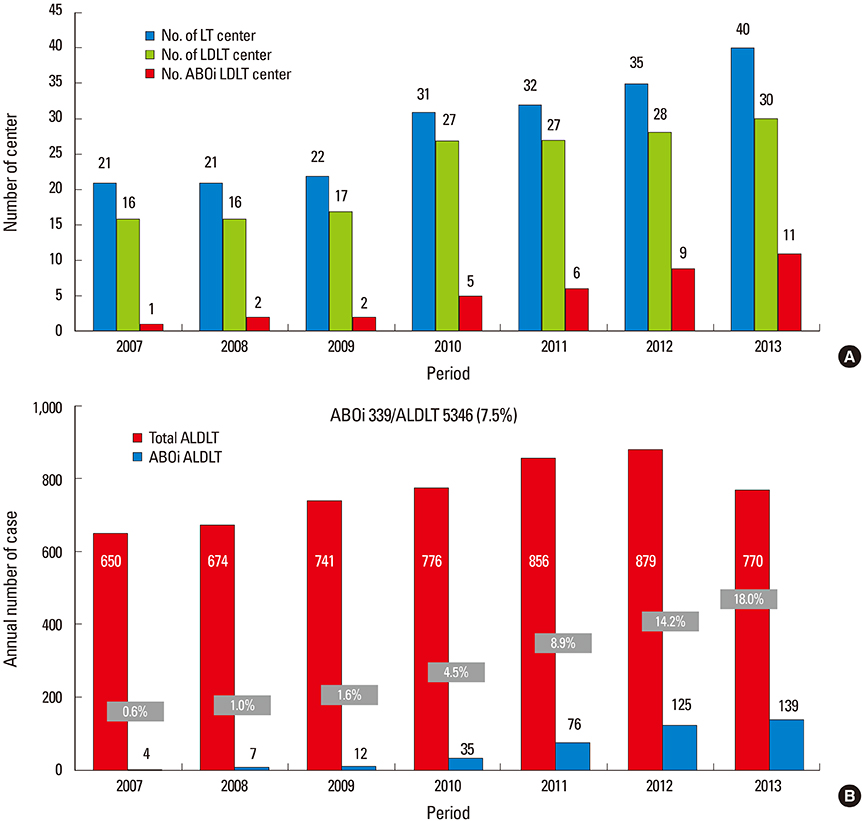
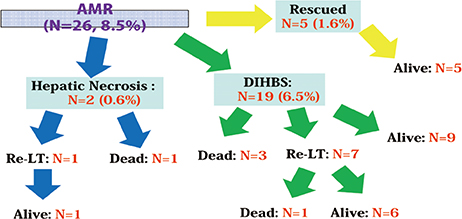
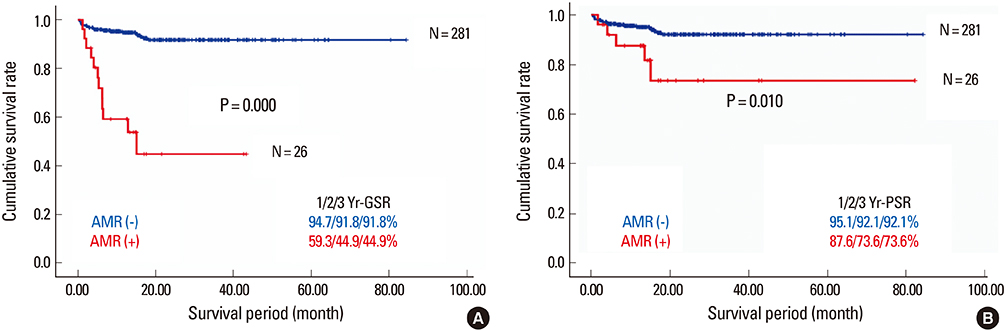
- Despite the great potential of ABO-incompatible (ABOi) liver transplantation (LT) for expanding the donor pool, serious concern about poor outcomes in the recipients has been a major obstacle to its widespread. The use of ABOi living donors is an attractive solution for expanding the liver donor pool, and various novel strategies for desensitization of ABO incompatibility have yielded promising results. The 1st breakthrough was local graft infusion therapy introduced by the Keio and Kyoto group; a second, epochal advance was the advent of the anti-CD20 monoclonal antibody, rituximab. Since then, the risk of fulminant hepatic necrosis caused by full-blown antibody-mediated rejection (AMR) has almost disappeared, and survival outcomes of ABOi LT have increased markedly. In the Korean experience, ABOi LT accounts for 18% of all adult living donor liver transplantation, and 3-year graft and patient survival rates are 86.5 and 87.6%, respectively. ABOi living donor LT is thus having a major impact on the donor pool and the recent achievements permit us to promote a nationwide ABOi LT program. However, concern still remains about diffuse intrahepatic biliary stricture (DIHBS), which is an attenuated form of AMR. Ultimately, we need to identify risk factors and preventive measures for this.
MeSH Terms
Figure
Cited by 3 articles
-
Cutting Edge Technologies in Organ Transplantation
Dongho Choi
Hanyang Med Rev. 2014;34(4):143-144. doi: 10.7599/hmr.2014.34.4.143.ABO-incompatible liver transplantation using only rituximab for patients with low anti-ABO antibody titer
Boram Lee, YoungRok Choi, Ho-Seong Han, Yoo-Seok Yoon, Jai Young Cho, Sook-Hyang Jeong, Jin-Wook Kim, Eun Sun Jang, Soomin Ahn
Ann Hepatobiliary Pancreat Surg. 2019;23(3):211-218. doi: 10.14701/ahbps.2019.23.3.211.Overcoming high pre-transplant isoagglutinin titers using high-dose intravenous immunoglobulin, salvage plasmapheresis, and booster rituximab without splenectomy in ABO-incompatible living donor liver transplantation: a case report
Hyung Hwan Moon
Kosin Med J. 2022;37(2):163-168. doi: 10.7180/kmj.21.036.
Reference
-
1. Kuramitsu K, Fukumoto T, Iwasaki T, Tominaga M, Matsumoto I, Ajiki T, et al. Long-term complications after liver transplantation. Transplant Proc. 2014; 46:797–803.
Article2. Gordon RD, Iwatsuki S, Esquivel CO, Tzakis A, Todo S, Starzl TE. Liver transplantation across ABO blood groups. Surgery. 1986; 100:342–348.3. Gugenheim J, Samuel D, Reynes M, Bismuth H. Liver transplantation across ABO blood group barriers. Lancet. 1990; 336:519–523.
Article4. Demetris AJ, Jaffe R, Tzakis A, Ramsey G, Todo S, Belle S, et al. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across ABO blood group barriers. Am J Pathol. 1988; 132:489–502.5. Tanaka A, Tanaka K, Kitai T, Yanabu N, Tokuka A, Sato B, et al. Living related liver transplantation across ABO blood groups. Transplantation. 1994; 58:548–553.
Article6. Tanabe M, Shimazu M, Wakabayashi G, Hoshino K, Kawachi S, Kadomura T, et al. Intraportal infusion therapy as a novel approach to adult ABO-incompatible liver transplantation. Transplantation. 2002; 73:1959–1961.
Article7. Nakamura Y, Matsuno N, Iwamoto H, Yokoyama T, Kuzuoka K, Kihara Y, et al. Successful case of adult ABO-incompatible liver transplantation: beneficial effects of intrahepatic artery infusion therapy: a case report. Transplant Proc. 2004; 36:2269–2273.
Article8. Usuda M, Fujimori K, Koyamada N, Fukumori T, Sekiguchi S, Kawagishi N, et al. Successful use of anti-CD20 monoclonal antibody (rituximab) for ABO-incompatible living-related liver transplantation. Transplantation. 2005; 79:12–16.
Article9. Egawa H, Ohdan H, Haga H, Tsuruyama T, Oike F, Uemoto S, et al. Current status of liver transplantation across ABO blood-type barrier. J Hepatobiliary Pancreat Surg. 2008; 15:131–138.
Article10. Egawa H, Teramukai S, Haga H, Tanabe M, Fukushima M, Shimazu M. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology. 2008; 47:143–152.
Article11. Tanabe M, Kawachi S, Obara H, Shinoda M, Hibi T, Kitagawa Y, et al. Current progress in ABO-incompatible liver transplantation. Eur J Clin Invest. 2010; 40:943–949.
Article12. Raut V, Uemoto S. Management of ABO-incompatible living-donor liver transplantation: past and present trends. Surg Today. 2011; 41:317–322.
Article13. Haga H, Egawa H, Shirase T, Miyagawa A, Sakurai T, Minamiguchi S, et al. Periportal edema and necrosis as diagnostic histological features of early humoral rejection in ABO-incompatible liver transplantation. Liver Transpl. 2004; 10:16–27.
Article14. Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, et al. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999; 10:2208–2214.15. Behr TM, Feucht HE, Richter K, Reiter C, Spes CH, Pongratz D, et al. Detection of humoral rejection in human cardiac allografts by assessing the capillary deposition of complement fragment C4d in endomyocardial biopsies. J Heart Lung Transplant. 1999; 18:904–912.
Article16. Magro CM, Pope Harman A, Klinger D, Orosz C, Adams P, Waldman J, et al. Use of C4d as a diagnostic adjunct in lung allograft biopsies. Am J Transplant. 2003; 3:1143–1154.
Article17. Haga H, Egawa H, Fujimoto Y, Ueda M, Miyagawa-Hayashino A, Sakurai T, et al. Acute humoral rejection and C4d immunostaining in ABO blood type-incompatible liver transplantation. Liver Transpl. 2006; 12:457–464.
Article18. Kozaki K, Egawa H, Ueda M, Oike F, Yoshizawa A, Fukatsu A, et al. The role of apheresis therapy for ABO incompatible living donor liver transplantation: the Kyoto University experience. Ther Apher Dial. 2006; 10:441–448.
Article19. Thalgahagoda S, Webb NJ, Roberts D, Birch A, Milford DV, Tavakoli A, et al. Successful ABO incompatible renal transplantation following rituximab and DFPP after failed immunoadsorption. Pediatr Transplant. 2014; 18:E74–E76.
Article20. Eskandary F, Wahrmann M, Biesenbach P, Sandurkov C, Konig F, Schwaiger E, et al. ABO antibody and complement depletion by immunoadsorption combined with membrane filtration--a randomized, controlled, cross-over trial. Nephrol Dial Transplant. 2014; 29:706–714.
Article21. de Weerd AE, van Agteren M, Leebeek FW, Ijzermans JN, Weimar W, Betjes MG. ABO-incompatible kidney transplant recipients have a higher bleeding risk after antigen-specific immunoadsorption. Transpl Int. Forthcoming 2014.
Article22. Ikegami T, Taketomi A, Soejima Y, Yoshizumi T, Uchiyama H, Harada N, et al. Rituximab, IVIG, and plasma exchange without graft local infusion treatment: a new protocol in ABO incompatible living donor liver transplantation. Transplantation. 2009; 88:303–307.
Article23. Song GW, Lee SG, Hwang S, Ahn CS, Kim KH, Moon DB, et al. Section 15. A desensitizing protocol without local graft infusion therapy and splenectomy is a safe and effective method in ABO-incompatible adult LDLT. Transplantation. 2014; 97:Suppl 8. S59–S66.
Article24. Raut V, Mori A, Kaido T, Ogura Y, Taku I, Nagai K, et al. Splenectomy does not offer immunological benefits in ABO-incompatible liver transplantation with a preoperative rituximab. Transplantation. 2012; 93:99–105.
Article25. Pescovitz MD. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006; 6:859–866.
Article26. Ramos EJ, Pollinger HS, Stegall MD, Gloor JM, Dogan A, Grande JP. The effect of desensitization protocols on human splenic B-cell populations in vivo. Am J Transplant. 2007; 7:402–407.
Article27. Toki D, Ishida H, Horita S, Setoguchi K, Yamaguchi Y, Tanabe K. Impact of low-dose rituximab on splenic B cells in ABO-incompatible renal transplant recipients. Transpl Int. 2009; 22:447–454.
Article28. Chung BH, Hong YA, Sun IO, Piao SG, Kim JI, Moon IS, et al. Determination of rituximab dose according to immunologic risk in ABO-incompatible kidney transplantation. Ren Fail. 2012; 34:974–979.
Article29. Egawa H, Teramukai S, Haga H, Tanabe M, Mori A, Ikegami T, et al. Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant. 2014; 14:102–114.
Article30. Egawa H, Oike F, Buhler L, Shapiro AM, Minamiguchi S, Haga H, et al. Impact of recipient age on outcome of ABO-incompatible living-donor liver transplantation. Transplantation. 2004; 77:403–411.
Article31. Kawagishi N, Satomi S. ABO-incompatible living donor liver transplantation: new insights into clinical relevance. Transplantation. 2008; 85:1523–1525.
Article32. Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, et al. Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era. J Hepatol. 2014; 61:575–582.
Article33. Uchiyama H, Mano Y, Taketomi A, Soejima Y, Yoshizumi T, Ikegami T, et al. Kinetics of anti-blood type isoagglutinin titers and B lymphocytes in ABO-incompatible living donor liver transplantation with rituximab and plasma exchange. Transplantation. 2011; 92:1134–1139.
Article34. Kim BW, Park YK, Kim YB, Wang HJ, Kim MW. Effects and problems of adult ABO-incompatible living donor liver transplantation using protocol of plasma exchange, intra-arterial infusion therapy, and anti-CD20 monoclonal antibody without splenectomy: case reports of initial experiences and results in Korea. Transplant Proc. 2008; 40:3772–3777.
Article35. Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, et al. Dual living donor liver transplantation with ABO-incompatible and ABO-compatible grafts to overcome small-for-size graft and ABO blood group barrier. Liver Transpl. 2010; 16:491–498.
Article36. Song GW, Lee SG, Hwang S, Ahn CS, Moon DB, Kim KH, et al. Successful experiences of ABO-incompatible adult living donor liver transplantation in a single institute: no immunological failure in 10 consecutive cases. Transplant Proc. 2013; 45:272–275.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ABO-Incompatible Living Donor Liver Transplantation
- ABO incompatibility is a risk factor for cytomegalovirus infection with living donor liver transplantation
- Immunologic strategies and outcomes in ABO-incompatible living donor liver transplantation
- The Diagnosis of Acute Antibody-Mediated Rejection in ABO-Incompatible Liver Transplants
- ABO-Incompatible Kidney Transplantation