Cancer Res Treat.
2011 Jun;43(2):102-107.
Surgical Outcomes of Hemorrhagic Metastatic Brain Tumors
- Affiliations
-
- 1Neuro-Oncology Clinic, National Cancer Center, Goyang, Korea. nslsh@ncc.re.kr
Abstract
- PURPOSE
Hemorrhagic metastatic brain tumors are not rare, but little is known about the surgical outcome following treatment. We conducted this study to determine the result of the surgical outcome of hemorrhagic metastatic brain tumors.
MATERIALS AND METHODS
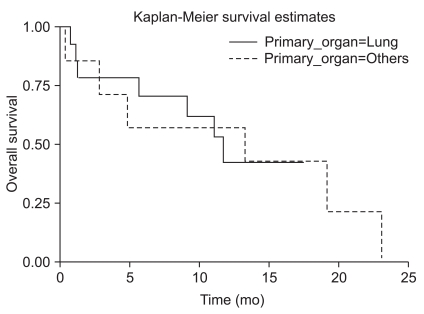
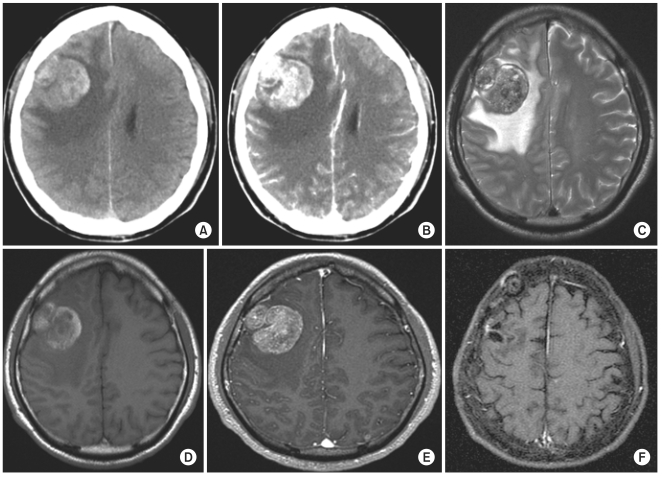
From July 2001 to December 2008, 21 patients underwent surgery for hemorrhagic metastatic brain tumors at our institution. 15 patients had lung cancer, 3 had hepatocellular carcinoma, and the rest had rectal cancer, renal cell carcinoma, and sarcoma. 20 patients had macroscopic hemorrhage in the tumors, and one patient had intracerebral hemorrhage surrounding the tumor. A retrospective clinical review was conducted focusing on the patterns of presenting symptoms and signs, as well as local recurrence following surgery.
RESULTS
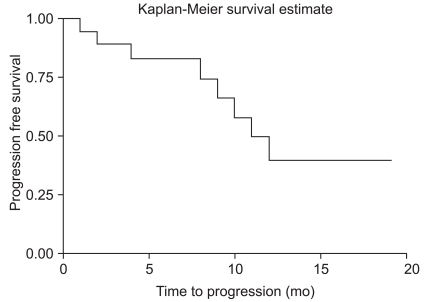
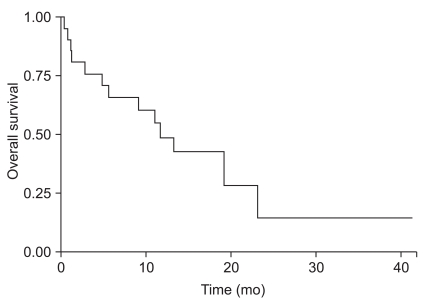
Among 21 hemorrhagic brain metastases, local recurrence developed in two patients. The 12 month progression free survival rate was 86.1%. Mean time to progression was 20.8 months and median survival time after surgery was 11.7 months.
CONCLUSION
The results of our study showed that hemorrhagic metastatic brain tumors rarely recurred after surgery. Surgery should be considered as a good treatment option for hemorrhagic brain metastasis, especially in cases with increased intracranial pressure or severe neurologic deficits.
MeSH Terms
Figure
Reference
-
1. Kondziolka D, Bernstein M, Resch L, Tator CH, Fleming JF, Vanderlinden RG, et al. Significance of hemorrhage into brain tumors: clinicopathological study. J Neurosurg. 1987; 67:852–857. PMID: 3316531.
Article2. Schrader B, Barth H, Lang EW, Buhl R, Hugo HH, Biederer J, et al. Spontaneous intracranial haematomas caused by neoplasms. Acta Neurochir (Wien). 2000; 142:979–985. PMID: 11086806.
Article3. Wakai S, Yamakawa K, Manaka S, Takakura K. Spontaneous intracranial hemorrhage caused by brain tumor: its incidence and clinical significance. Neurosurgery. 1982; 10:437–444. PMID: 7099393.4. Cheng MH, Lin JW. Intracranial meningioma with intratumoral hemorrhage. J Formos Med Assoc. 1997; 96:116–120. PMID: 9071837.5. Cheng SY, Nagane M, Huang HS, Cavenee WK. Intracerebral tumor-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc Natl Acad Sci U S A. 1997; 94:12081–12087. PMID: 9342366.
Article6. Liwnicz BH, Wu SZ, Tew JM Jr. The relationship between the capillary structure and hemorrhage in gliomas. J Neurosurg. 1987; 66:536–541. PMID: 3031239.
Article7. Maiuri F, D'Andrea F, Gallicchio B, Carandente M. Intracranial hemorrhages in metastatic brain tumors. J Neurosurg Sci. 1985; 29:37–41. PMID: 4067634.8. Lavine SD, Petrovich Z, Cohen-Gadol AA, Masri LS, Morton DL, O'Day SJ, et al. Gamma knife radiosurgery for metastatic melanoma: an analysis of survival, outcome, and complications. Neurosurgery. 1999; 44:59–64. PMID: 9894964.
Article9. Redmond AJ, Diluna ML, Hebert R, Moliterno JA, Desai R, Knisely JP, et al. Gamma Knife surgery for the treatment of melanoma metastases: the effect of intratumoral hemorrhage on survival. J Neurosurg. 2008; 109(Suppl):99–105. PMID: 19123895.
Article10. Seung SK, Sneed PK, McDermott MW, Shu HK, Leong SP, Chang S, et al. Gamma knife radiosurgery for malignant melanoma brain metastases. Cancer J Sci Am. 1998; 4:103–109. PMID: 9532412.11. van den Doel EM, van Merriënboer FJ, Tulleken CA. Cerebral hemorrhage from unsuspected choriocarcinoma. Clin Neurol Neurosurg. 1985; 87:287–290. PMID: 4092410.
Article12. Kidd D, Plant GT, Scaravilli F, McCartney AC, Stanford M, Graham EM. Metastatic choriocarcinoma presenting as multiple intracerebral haemorrhages: the role of imaging in the elucidation of the pathology. J Neurol Neurosurg Psychiatry. 1998; 65:939–941. PMID: 9854978.
Article13. Iwama T, Ohkuma A, Miwa Y, Sugimoto S, Itoh T, Takada M, et al. Brain tumors manifesting as intracranial hemorrhage. Neurol Med Chir (Tokyo). 1992; 32:130–135. PMID: 1377794.
Article14. Hirano A, Matsui T. Vascular structures in brain tumors. Hum Pathol. 1975; 6:611–621. PMID: 1100515.
Article15. McCormick WF, Ugajin K. Fatal hemorrhage into a medulloblastoma: case report. J Neurosurg. 1967; 26:78–81. PMID: 6018785.16. Scatliff JH, Radcliffe WB, Pittman HH, Park CH. Vascular structure of glioblastomas. Am J Roentgenol Radium Ther Nucl Med. 1969; 105:795–805.
Article17. Weller RO, Foy M, Cox S. The development and ultrastructure of the microvasculature in malignant gliomas. Neuropathol Appl Neurobiol. 1977; 3:307–322.
Article18. Jung S, Moon KS, Jung TY, Kim IY, Lee YH, Rhu HH, et al. Possible pathophysiological role of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in metastatic brain tumor-associated intracerebral hemorrhage. J Neurooncol. 2006; 76:257–263. PMID: 16158215.
Article19. Armstrong JG, Wronski M, Galicich J, Arbit E, Leibel SA, Burt M. Postoperative radiation for lung cancer metastatic to the brain. J Clin Oncol. 1994; 12:2340–2344. PMID: 7964950.
Article20. Burt M, Wronski M, Arbit E, Galicich JH. Resection of brain metastases from non-small-cell lung carcinoma: results of therapy. Memorial Sloan-Kettering Cancer Center Thoracic Surgical Staff. J Thorac Cardiovasc Surg. 1992; 103:399–410. PMID: 1312184.21. Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998; 280:1485–1489. PMID: 9809728.
Article22. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990; 322:494–500. PMID: 2405271.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stereotactic radiosurgery for brain metastases
- Pathophysiological Role of Vascular Endothelial Growth Factor (VEGF) and Matrix Metalloproteinases (MMP) in Metastatic Brain Tumor-associated Intracerebral Hemorrhage
- Regional Cerebral Blood Flow in Intracranial Tumors
- A Clinical Analysis of Metastatic Brain Tumors
- Concomitant Subdural Hemorrhage and Intracerebral Hemorrhage due to Brain Metastasis of the Hepatocellular Carcinoma