Ann Surg Treat Res.
2015 Apr;88(4):200-207. 10.4174/astr.2015.88.4.200.
Ki-67 and p53 expression as a predictive marker for early postoperative recurrence in pancreatic head cancer
- Affiliations
-
- 1Department of Trauma Surgery, Pusan National University Hospital, Busan, Korea. wkafyddl@hanmail.net
- 2Department of Pathology, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Surgery, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2167016
- DOI: http://doi.org/10.4174/astr.2015.88.4.200
Abstract
- PURPOSE
This study aimed to evaluate the clinical significance of Ki-67 and p53 expressions in patients with pancreatic head cancer.
METHODS
Between May 2008 and April 2013, immunohistochemical staining for Ki-67 and p53 was performed in 34 patients with pancreatic head cancer (ductal adenocarcinoma). All 34 patients underwent pancreaticoduodenectomy at Chonnam National University Hwasun Hospital, Hwasun, Korea. Clinical and histopathological characteristics were analyzed, relative to p53 expression.
RESULTS
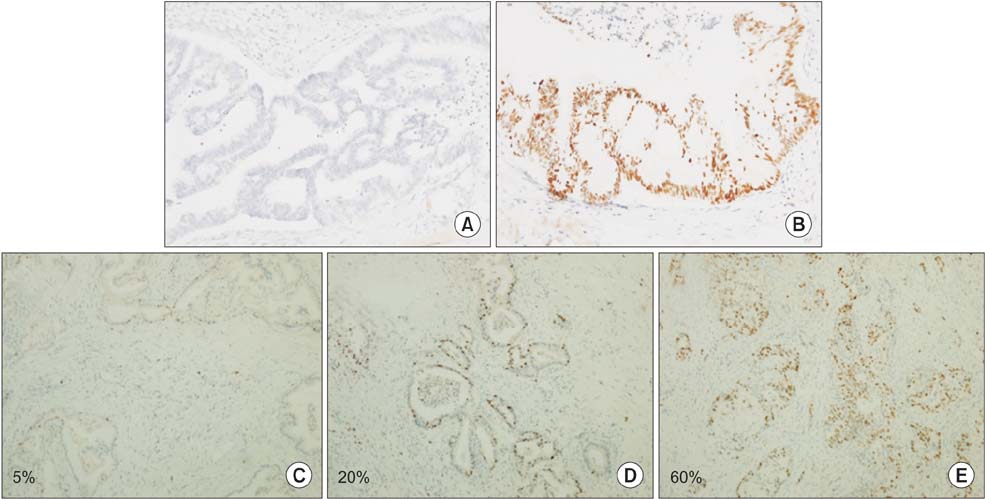
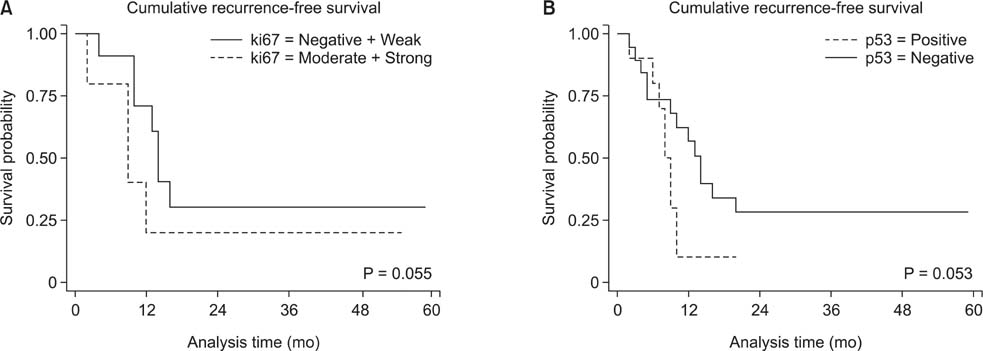
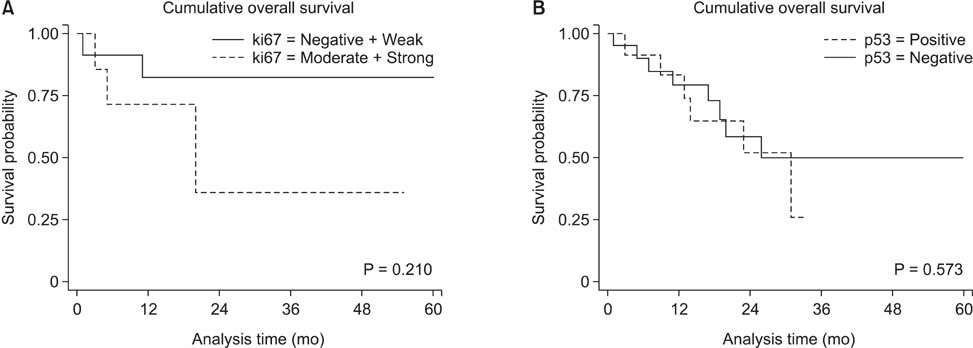
Thirty (88.2%) and twenty-one (61.7%) of the 34 pancreatic head cancers exhibited positive expression of Ki-67 and p53, respectively. Patients expressing Ki-67 and p53 experienced more frequent tumor recurrences within 1 year after surgical resection (P = 0.003 and P = 0.030, respectively). However, no correlation was detected between Ki-67 and p53 expression. Ki-67 expression was correlated with pathological grade, lymph node metasatsis, and clinical stage (P < 0.05). Importantly, Ki-67 was the independent predictive factor for postoperative recurrence within 1 year in both univariable and multivariable analyses (odds ratio, 27.219; 95% confidence interval, 1.403-528.135; P = 0.029).
CONCLUSION
The expression of Ki-67 and p53 are significantly related to early postoperative recurrence within 1 year after surgical resection in pancreatic head cancer. Especially, Ki-67 was the independent predictive factor for postoperative recurrence within 1 year. Therefore, immunohistochemical staining for Ki-67 and p53 may be applied as a predictive marker for early postoperative recurrence in pancreatic head cancer.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Clinical significance of pancreatic intraepithelial neoplasia in resectable pancreatic cancer on survivals
Da-Young Yu, Young-Dong Yu, Wan-Bae Kim, Hyung-Joon Han, Sae-Byul Choi, Dong-Sik Kim, Sang-Yong Choi, Joo-Young Kim, Hyeyoon Chang, Baek-Hui Kim
Ann Surg Treat Res. 2018;94(5):247-253. doi: 10.4174/astr.2018.94.5.247.
Reference
-
1. Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992; 326:455–465.2. Jensen OM, Esteve J, Moller H, Renard H. Cancer in the European Community and its member states. Eur J Cancer. 1990; 26:1167–1256.3. Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000; 6:2969–2972.4. Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000; 182:311–322.5. Jalava P, Kuopio T, Juntti-Patinen L, Kotkansalo T, Kronqvist P, Collan Y. Ki67 immunohistochemistry: a valuable marker in prognostication but with a risk of misclassification: proliferation subgroups formed based on Ki67 immunoreactivity and standardized mitotic index. Histopathology. 2006; 48:674–682.6. Gentile V, Vicini P, Giacomelli L, Cardillo MR, Pierangeli A, Degener AM. Detection of human papillomavirus DNA, p53 and ki67 expression in penile carcinomas. Int J Immunopathol Pharmacol. 2006; 19:209–215.7. Lee CS. Differences in cell proliferation and prognostic significance of proliferating cell nuclear antigen and Ki-67 antigen immunoreactivity in in situ and invasive carcinomas of the extrahepatic biliary tract. Cancer. 1996; 78:1881–1887.8. Dong M, Dong Q, Zhang H, Zhou J, Tian Y, Dong Y. Expression of Gadd45a and p53 proteins in human pancreatic cancer: potential effects on clinical outcomes. J Surg Oncol. 2007; 95:332–336.9. Scarpa A, Capelli P, Mukai K, Zamboni G, Oda T, Iacono C, et al. Pancreatic adenocarcinomas frequently show p53 gene mutations. Am J Pathol. 1993; 142:1534–1543.10. Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997; 57:1731–1734.11. Kawesha A, Ghaneh P, Andren-Sandberg A, Ograed D, Skar R, Dawiskiba S, et al. K-ras oncogene subtype mutations are associated with survival but not expression of p53, p16(INK4A), p21(WAF-1), cyclin D1, erbB-2 and erbB-3 in resected pancreatic ductal adenocarcinoma. Int J Cancer. 2000; 89:469–474.12. Zhang SY, Ruggeri B, Agarwal P, Sorling AF, Obara T, Ura H, et al. Immunohistochemical analysis of p53 expression in human pancreatic carcinomas. Arch Pathol Lab Med. 1994; 118:150–154.13. Dong M, Ma G, Tu W, Guo KJ, Tian YL, Dong YT. Clinicopathological significance of p53 and mdm2 protein expression in human pancreatic cancer. World J Gastroenterol. 2005; 11:2162–2165.14. Bold RJ, Hess KR, Pearson AS, Grau AM, Sinicrope FA, Jennings M, et al. Prognostic factors in resectable pancreatic cancer: p53 and bcl-2. J Gastrointest Surg. 1999; 3:263–277.15. Salek C, Minarikova P, Benesova L, Nosek V, Strnad R, Zavoral M, et al. Mutation status of K-ras, p53 and allelic losses at 9p and 18q are not prognostic markers in patients with pancreatic cancer. Anticancer Res. 2009; 29:1803–1810.16. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer;2010.17. Stanton KJ, Sidner RA, Miller GA, Cummings OW, Schmidt CM, Howard TJ, et al. Analysis of Ki-67 antigen expression, DNA proliferative fraction, and survival in resected cancer of the pancreas. Am J Surg. 2003; 186:486–492.18. Hu HY, Liu H, Zhang JW, Hu K, Lin Y. Clinical significance of Smac and Ki-67 expression in pancreatic cancer. Hepatogastroenterology. 2012; 59:2640–2643.19. Shimamura A, Fisher DE. p53 in life and death. Clin Cancer Res. 1996; 2:435–440.20. Nakamori S, Yashima K, Murakami Y, Ishikawa O, Ohigashi H, Imaoka S, et al. Association of p53 gene mutations with shor t survival in pancreat ic adenocarcinoma. Jpn J Cancer Res. 1995; 86:174–181.21. Lynch HT, Brand RE, Lynch JF, Fusaro RM, Kern SE. Hereditary factors in pancreatic cancer. J Hepatobiliary Pancreat Surg. 2002; 9:12–31.22. Ruggeri BA, Huang L, Berger D, Chang H, Klein-Szanto AJ, Goodrow T, et al. Molecular pathology of primary and metastatic ductal pancreatic lesions: analyses of mutations and expression of the p53, mdm-2, and p21/WAF-1 genes in sporadic and familial lesions. Cancer. 1997; 79:700–716.23. Yokoyama M, Yamanaka Y, Friess H, Buchler M, Korc M. p53 expression in human pancreatic cancer correlates with enhanced biological aggressiveness. Anticancer Res. 1994; 14(6B):2477–2483.24. Sato Y, Nio Y, Song MM, Sumi S, Hirahara N, Minari Y, et al. p53 protein expression as prognostic factor in human pancreatic cancer. Anticancer Res. 1997; 17(4A):2779–2788.25. Luttges J, Zamboni G, Kloppel G. Recommendation for the examination of pancreaticoduodenectomy specimens removed from patients with carcinoma of the exocrine pancreas. A proposal for a standardized pathological staging of pancreaticoduodenectomy specimens including a checklist. Dig Surg. 1999; 16:291–296.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of p53 and Ki-67 Expression in Colorectal Cancer
- The Prognostic Value of the S-phase Fraction and Ki-67 Expression in Early Breast Cancer
- Correlation between p53, PCNA and Ki-67 Expression in Head Neck Squamous Cell Carcinoma
- Study of the Correlation Among p53 Expression, Epidermal Hyperplasia, Dermal Inflammation, p21 Expression, and Ki-67 Expression in the Actinic Keratosis
- Immunohistochemical Markers for Metastasis in Clear Cell Renal Cell Carcinoma