Cancer Res Treat.
2005 Dec;37(6):339-343.
The Safety and Efficacy of Second-line Single Docetaxel (75 mg/m2) Therapy in Advanced Non-Small Cell Lung Cancer Patients who were Previously Treated with Platinum-based Chemotherapy
- Affiliations
-
- 1Department of Internal Medicine, Lung Cancer Center, St. Vincent's Hospital, Suwon, Korea. kimhoonkyo@yahoo.co.kr
- 2Department of Diagnostic Radiology, Lung Cancer Center, St. Vincent's Hospital, Suwon, Korea.
- 3Department of Radiation Oncology, Lung Cancer Center, St. Vincent's Hospital, Suwon, Korea.
- 4Department of Chest Surgery, Lung Cancer Center, St. Vincent's Hospital, Suwon, Korea.
- 5Department of Pathology, Lung Cancer Center, St. Vincent's Hospital, Suwon, Korea.
Abstract
- PURPOSE
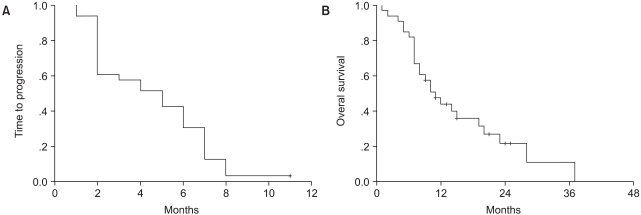
When used in the second-line setting, single- agent chemotherapy has produced response rates of more than 10% or median survival times greater than 4 months. We studied the safety and efficacy of using second-line single docetaxel (75 mg/m2) for advanced NSCLC patients who were previously treated with platinum-based chemotherapy in Korea. MATERIALS AND METHODS: Thirty-three patients with advanced NSCLC received chemotherapy from May 2002 to January 2005. We retrospectively reviewed the charts of these patients. The patients received 75 mg/m2 of doxetaxel on day 1 and this was repeated at 3-week intervals. RESULTS: The median age was 63 years (range: 42~77 years); 16 patients had adenocarcinoma and 8 patients had squamous cell carcinoma. The median number of cycles was 4 (range: 1~7 cycles). Of the 33 patients, 6 patients had partial responses, 13 patients had stable disease and 14 patients had progressive disease. The response rate was 18.2%. The median overall survival was 11 months (range: 7~15 months), and the median progression free survival was 5 months (range: 3~7 months). The median response duration was 5 months (range: 4~9 months). A total of 137 cycles were evaluated for toxicity. We observed grade 3 or 4 neutropenia in 79 cycles (57.6%), grade 3 or 4 leukopenia in 46 cycles (33.6%), and grade 3 febrile neutropenia in 2 cycles (1.5%). The median nadir day was day 9 (range: day 5~19), and the median number of G-CSF injections was 2 (range: 0~6). The most common non-hematologic toxicities were myalgia/arthralgia and neurotoxicity, but any grade 3 or 4 non-hematologic toxicity was not observed. The major toxicity of this therapy was neutropenia. The absolute neutrophil count decreased relatively rapidly, but neutropenic fever or related infection was rare. There were no treatment-related deaths. CONCLUSION: These results revealed a satisfactory response rate (18.2%) with using docetaxel as the second- line chemotherapy for NSCLC. The second-line docetaxel was an active and well-tolerated regimen in patients with advanced NSCLC pretreated with platinum-based chemotherapy.
MeSH Terms
Figure
Reference
-
1. Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomized clinical trials. BMJ. 1995; 311:899–909. PMID: 7580546.2. American Society of Clinical Oncology Group. Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. J Clin Oncol. 1997; 15:2996–3018. PMID: 9256144.3. Fossella FV, Lee JS, Hong WK. Management strategies for recurrent non-small cell lung cancer. Semin Oncol. 1997; 24:455–462. PMID: 9280225.4. Belani CP. Single agents in the second-line treatment of non-small cell lung cancer. Semin Oncol. 1998; 25:10–14. PMID: 9704670.5. Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, et al. The TAX 320 Non-Small Cell Lung Cancer Study Group. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol. 2000; 18:2354–2362. PMID: 10856094.
Article6. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000; 18:2095–2103. PMID: 10811675.
Article7. Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S Jr, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004; 22:330–353. PMID: 14691125.
Article8. Miller AB, Hoodgstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981; 47:207–214. PMID: 7459811.
Article9. Crino L, Mosconi AM, Scagliotti G, Selvaggi G, Novello S, Rinaldi M, et al. Gemcitabine as second-line treatment for advanced non-small-cell lung cancer: a phase II trial. J Clin Oncol. 1999; 17:2081–2085. PMID: 10561261.10. Sculier JP, Berghmans T, Lafitte JJ, Richez M, Recloux P, Van Cutsem O, et al. A phase II study testing paclitaxel as second-line single agent treatment for patients with advanced non-small cell lung cancer failing after a first-line chemotherapy. Lung Cancer. 2002; 37:73–77. PMID: 12057870.
Article11. Pronzato P, Landucci M, Vaira F, Vigani A, Bertelli G. Failure of vinorelbine to produce responses in pretreated non-small cell lung cancer patients. Anticancer Res. 1994; 14:1413–1415. PMID: 8067715.12. Kosmas C, Tsavaris N, Panopoulos C, Vadiaka M, Stavroyianni N, Kourelis T, et al. Gemcitabine and vinorelbine as second-line therapy in non-small-cell lung cancer after prior treatment with taxane+platinum-based regimens. Eur J Cancer. 2001; 37:972–978. PMID: 11334721.
Article13. Chang AY, DeVore R, Johnson D. Pilot study of vinorelbine (navelbine) and paclitaxel in patients with refractory non-small cell lung cancer. Semin Oncol. 1996; 23(Suppl 5):19–21. PMID: 8610231.14. Androulakis N, Kouroussis C, Kakolyris S, Tzannes S, Papadakis E, Papadimitriou C, et al. Salvage treatment with paclitaxel and gemcitabine for patients with non-small-cell lung cancer after cisplatin- or docetaxel-based chemotherapy: a multicenter phase II study. Ann Oncol. 1998; 9:1127–1130. PMID: 9834827.
Article15. Lee GW, Kang JH, Kim SH, Lee HY, Kim HC, Lee WS, et al. A phase II trial of docetaxel and ifosfamide for patients with platinum-resistant or refractory non-small cell lung cancer in a salvage setting. Cancer Res Treat. 2004; 36:287–292.
Article16. Gandara DR, Vokes E, Green M, Bonomi P, Devore R, Comis R, et al. Activity of docetaxel in platinum-treated non-small-cell lung cancer: results of a phase II multicenter trial. J Clin Oncol. 2000; 18:131–135. PMID: 10623703.17. Alexopoulos K, Kouroussis C, Androulakis N, Papadakis E, Vaslamatzis M, Kakolyris S, et al. Docetaxel and granulocyte colony-stimulating factor in patients with advanced non-small-cell lung cancer previously treated with platinum-based chemotherapy: a multicenter phase II trial. Cancer Chemother Pharmacol. 1999; 43:257–262. PMID: 9923557.
Article18. Fossella FV, Lee JS, Shin DM, Calayag M, Huber M, Perez-Soler R, et al. Phase II study of docetaxel for advanced or metastatic platinum-refractory non-small-cell lung cancer. J Clin Oncol. 1995; 13:645–651. PMID: 7884425.
Article19. Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003; 290:2149–2158. PMID: 14570950.20. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial). J Clin Oncol. 2003; 21:2237–2246. PMID: 12748244.21. Thatcher N, Chang A, Parikh P, Pemberton K, Archer V. ISEL: A phase III survival study comparing gefitinib (IRESSA) plus best supportive care (BSC) with placebo plus BSC in patients with advanced non-small cell lung cancer who had received one or two prior chemotherapy regimens. Lung Cancer. 2005; 49:S4.22. Lee DH, Han JY, Lee HG, Lee JJ, Lee EK, Kim HY, et al. Gefitinib as a first-line therapy of advanced or metastatic adenocarcinoma of the lung in never-smokers. Clin Cancer Res. 2005; 11:3032–3037. PMID: 15837758.
Article23. Smit EF, Mattson K, von Pawel J, Menegold C, Clarke S, Postmus PE. ALIMTA® (pemetrexed disodium) as second-line treatment of non-small cell lung cancer: A phase II study. Ann Oncol. 2003; 21:2636–2644.24. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004; 22:1589–1597. PMID: 15117980.
Article25. Nakamura Y, Kunitoh H, Kubota K, Sekine I, Yamamoto N, Tamura T, et al. Retrospective analysis of safety and efficacy of low-dose docetaxel 60 mg/m2 in advanced non-small cell lung cancer patients previously treated with platinum-based chemotherapy. Am J Clin Oncol. 2003; 26:459–464. PMID: 14528070.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Docetaxel as Second-line Monotherapy for Advanced Non-small Cell Lung Cancer
- A Phase II Trial of Docetaxel and Ifosfamide for Patients with Platinum-Resistant or Refractory Non-Small Cell Lung Cancer in a Salvage Setting
- Efficacy of Combination Chemotherapy with Paclitaxel and Cisplatin in Patients with Advanced Non-Small Cell Lung Cancer
- The efficacy and toxicity of docetaxel in patients with recurrent or persistent epithelial ovarian cancer
- Effect of Combination Chemotherapy with Docetaxel Plus Cisplatin in Patients with Advanced Non-Small Cell Lung Cancer