Cancer Res Treat.
2010 Jun;42(2):107-114.
The Keratin-14 Expression in Actinic Keratosis and Squamous Cell Carcinoma: Is This a Prognostic Factor for Tumor Progression?
- Affiliations
-
- 1Department of Dermatology, College of Medicine, The Catholic University of Korea, Seoul, Korea. dervint@catholic.ac.kr
Abstract
- PURPOSE
Actinic keratosis (AK) is an incipient form of cutaneous squamous cell carcinoma (SCC). We determined if the pattern of expression of keratin-14 (K14) is a factor for tumor progression in AK and SCC.
MATERIALS AND METHODS
Eighteen sections from the tissues of 16 patients were stained with anti-K14 antibody and p16(INK4a). Among the 16 patients, 4 were diagnosed with both SCC and AK at the same site, but AK developed first and SCC developed subsequently. Thus, SCC may have evolved from AK. The other 12 patients were only diagnosed with AK.
RESULTS
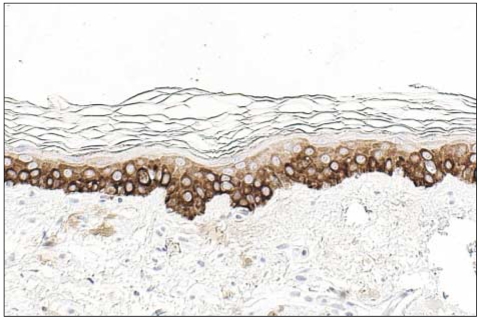
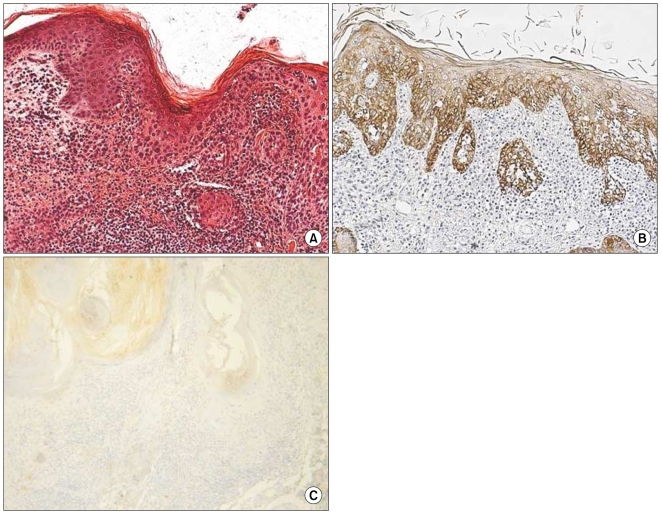
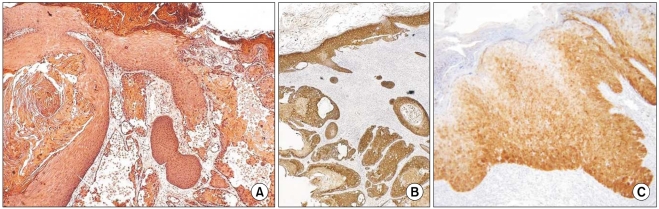
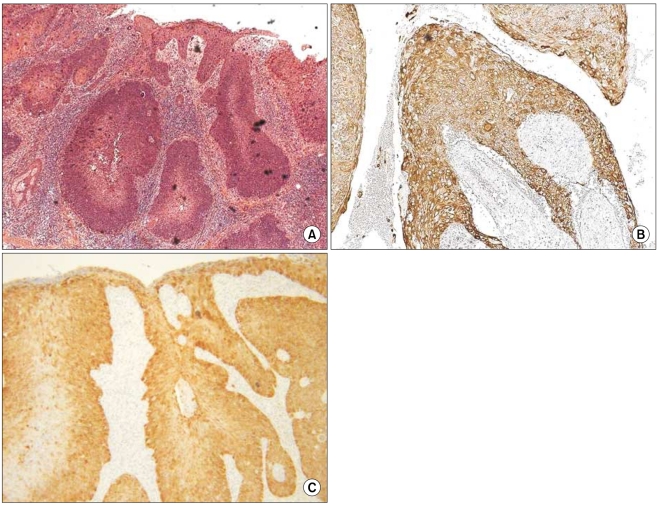
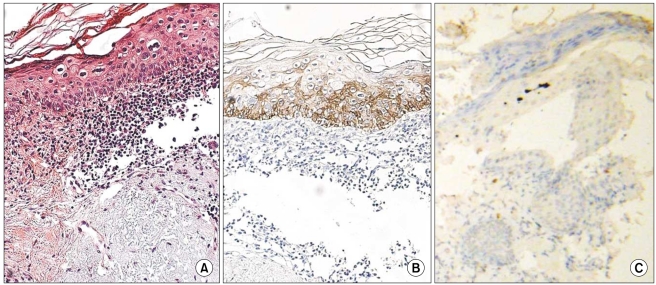
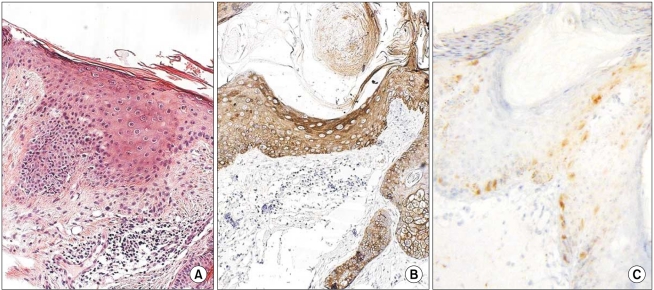
In all of the AK and SCC tissues, basement membranes showed positive staining for K14. However, strong reactivities were shown in the spinous and granular layers and focuses of dermal invasion in the SCC tissues developed from AK. Two and 3 of the 12 AK cases had moderately positive reactions for K14 in the spinous and granular layers, respectively. Also, all SCC tissues except one had moderate-to-strong reactions in the basal, spinous, and granular layers for p16(INK4a). Two of the 12 AK cases had weak-to-moderate positive reactions in the basal, spinous, and horny layers for p16(INK4a).
CONCLUSION
The results of our study advance our understanding of the pathogenesis of SCC developing from AK. The results also indicate a differential role in the control of K14 in normal epithelia, AK, and SCC. K14 expression in the spinous and granular layers may be a prognostic factor for tumor progression of AK.
Keyword
MeSH Terms
Figure
Reference
-
1. Duncan OK, Geisse JK. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Epithelial precancerous lesions. Fitzpatrick's dermatology in general medicine. 2007. 7th ed. New York: McGraw-Hill;p. 1007–1028.2. Giles GG, Marks R, Foley P. Incidence of non-melanocytic skin cancer treated in Australia. Br Med J (Clin Res Ed). 1988; 296:13–17.
Article3. Fukamizu H, Inoue K, Matsumoto K, Okayama H, Moriguchi T. Metastatic squamous-cell carcinomas derived from solar keratosis. J Dermatol Surg Oncol. 1985; 11:518–522. PMID: 3998264.
Article4. Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000; 42:23–24. PMID: 10607353.
Article5. Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002; 40:403–439. PMID: 12010363.
Article6. Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: pattern of expression in normal epithelia, tumors and cultured cells. Cell. 1982; 31:11–24. PMID: 6186379.7. Chu PG, Lyda MH, Weiss LM. Cytokeratin 14 expression in epithelial neoplasms: a survey of 435 cases with emphasis on its value in differentiating squamous cell carcinomas from other epithelial tumours. Histopathology. 2001; 39:9–16. PMID: 11454039.
Article8. Salama ME, Mahmood MN, Qureshi HS, Ma C, Zabro RJ, Ormsby AH. p16INK4a expression in actinic keratosis and Bowen's disease. Br J Dermatol. 2003; 149:1006–1012. PMID: 14632806.9. Choi YD, Park JN, Kang MS, Cho SH, Park SW. Expression of cytokeratin and Ki-67 in development of the pilomatricoma. Korean J Dermatol. 2003; 41:1619–1626.10. Anwar J, Wrone DA, Kimyai-Asadi A, Alam M. The development of actinic keratosis into invasive squamous cell carcinoma: Evidence and evolving classification schemes. Clin Dermatol. 2004; 22:189–196. PMID: 15262304.
Article11. Fu W, Cockerell CJ. The actinic (solar) keratosis: a 21st century perspective. Arch Dermatol. 2003; 139:66–70. PMID: 12533168.12. Lober BA, Lober CW. Actinic keratosis is squamous cell carcinoma. South Med J. 2000; 93:650–655. PMID: 10923948.
Article13. Hurwitz RM, Monger LE. Solar keratosis: an evolving squamous cell carcinoma. Benign or maligmamt? Dermatol Surg. 1995; 21:184. PMID: 7894943.14. Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squmaous cell carcinoma. Lancet. 1988; 1:795–797. PMID: 2895318.15. Harnden P, Southgate J. Cytokerain 14 as a marker of squmaous differention in transitional cell carcinoma. J Clin Pathol. 1997; 50:1032–1033. PMID: 9516889.16. Hudson AR, Smoller BR. Update on dermatopathology. Dermatol Clin. 1999; 17:667–689. PMID: 10410866.17. Fuchs E, Weber K. Intermediated filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994; 63:345–382. PMID: 7979242.18. Jiang CK, Tomic-Canic M, Lucas DJ, Simon M, Blumenberg M. TGF beta promotes the basal phenotype of epidermal keratinocytes: transcriptional induction of K5 and K14 keratin genes. Growth Factors. 1995; 12:87–97. PMID: 8679251.19. Komine M, Okinaga M, Takeda F, Nashiro K, Kikuchi K, Murakami T, et al. Patterns of basal cell keratin 14 expression in Bowen's disease: a possible marker for tumor progression. Br J Dermatol. 2001; 145:223–228. PMID: 11531783.20. Marley JJ, Robinson PA, Hume WJ. Expression of human cytokeratin 14 in normal, premalignant and malignant oral tissue following isolation by plaque differential hybridization. Eur J Cancer B Oral Oncol. 1994; 30B:305–311. PMID: 7535610.21. Su L, Morgan PR, Lane EB. Keratin 14 and 19 expression in normal, dysplastic and malignant oral epithelia. A study using in situ hybridization and immunohistochemistry. J Oral Pathol Med. 1996; 25:293–301. PMID: 8887072.22. Piccinin S, Doglioni C, Maestro R, Vukosavljevic T, Gasparotto D, D'Orazi C, et al. p16/CDKN2 and CDK4 gene mutations in sporadic melanoma development and progression. Int J Cancer. 1997; 74:26–30. PMID: 9036865.23. Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, et al. Overexpression of p16INK4A as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001; 92:276–284. PMID: 11291057.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Preliminary Study of Apoptosis and Expression of p53, bcl-2, PCNA and iNOS in Porokeratosis and Actinic Keratosis
- Pigmented Squamous Cell Carcinoma Arising from Pigmented Actinic Keratosis
- Cutaneous Horn in Premalignant and Malignant Conditions
- The Expression of c-Jun and JunB in Various Skin Tumors
- Basal Cell Carcinoma Arising from the Scar of Laser Ablation for a Pre-Existing Actinic Keratosis