Allergy Asthma Immunol Res.
2016 Jul;8(4):375-382. 10.4168/aair.2016.8.4.375.
Autologous Immunoglobulin Therapy in Patients With Severe Recalcitrant Atopic Dermatitis: Long-Term Changes of Clinical Severity and Laboratory Parameters
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. dhnahm@gmail.com
- KMID: 2165921
- DOI: http://doi.org/10.4168/aair.2016.8.4.375
Abstract
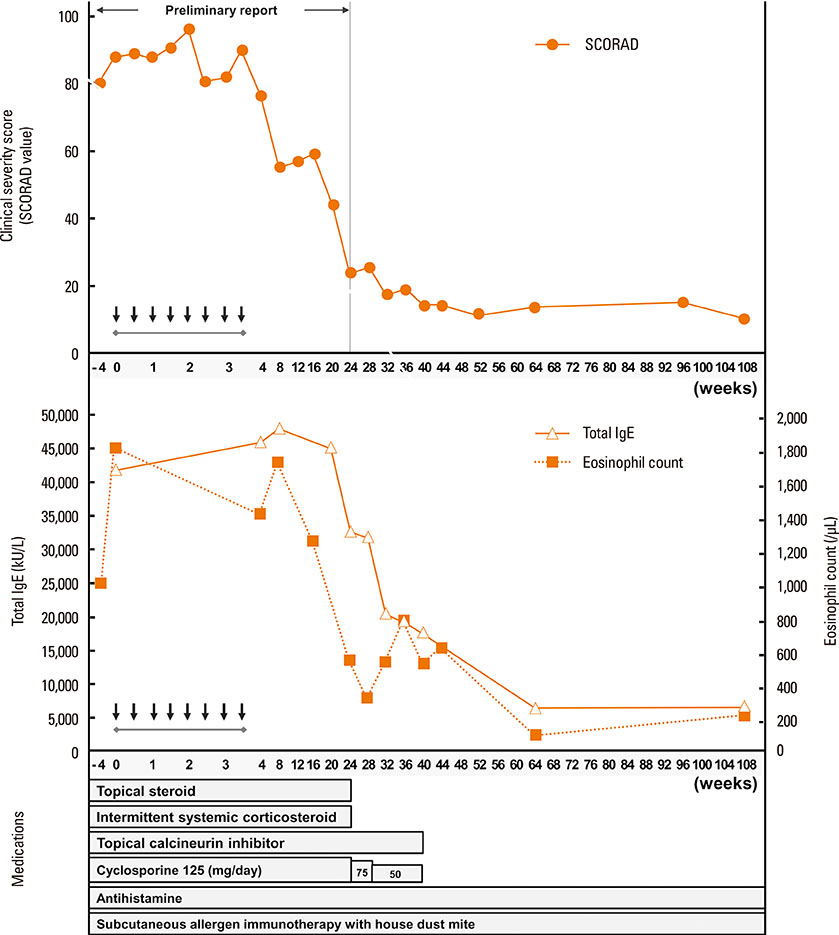
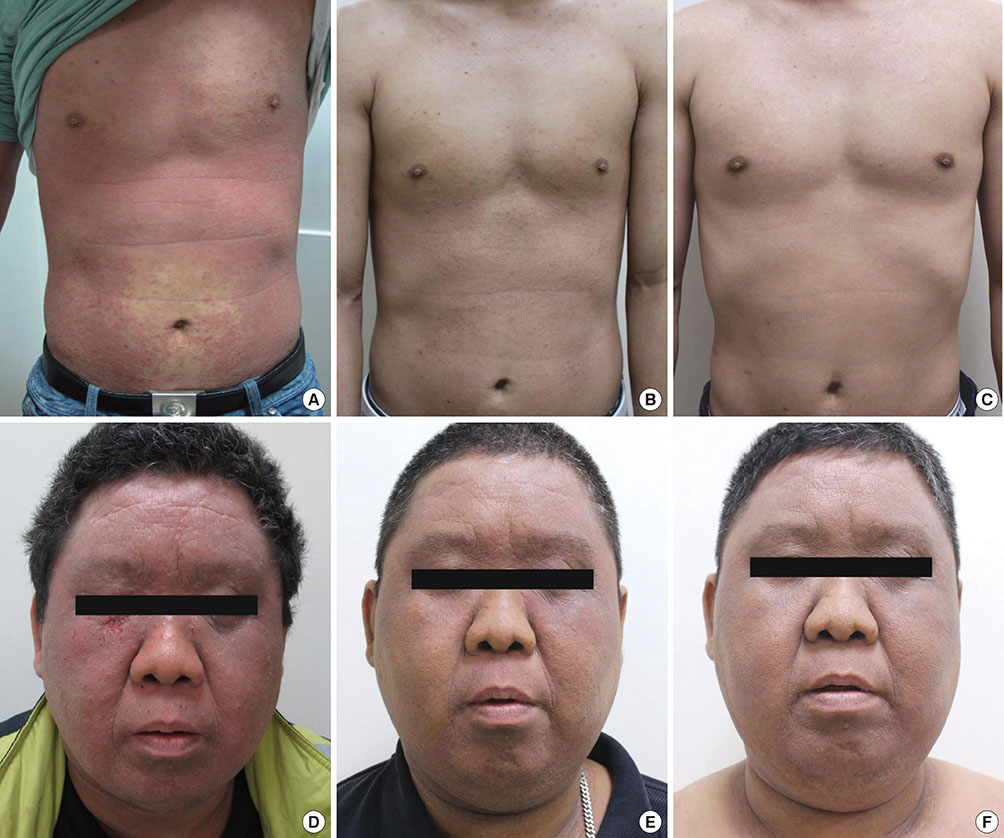
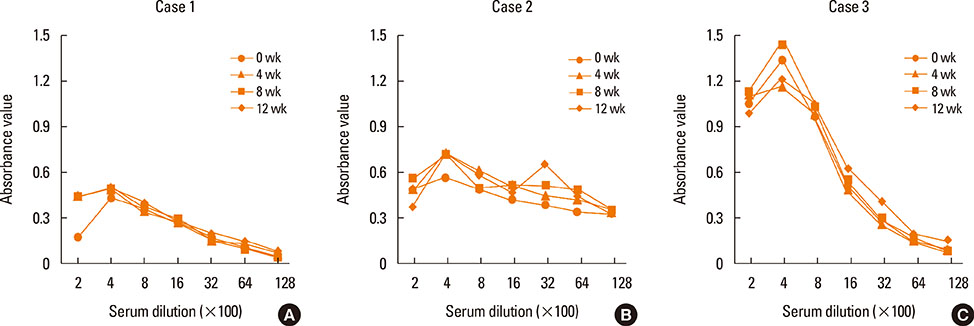
- This report evaluated long-term changes in clinical severity and laboratory parameters in 3 adult patients with severe recalcitrant atopic dermatitis (AD) who were treated with intramuscular injections of 50 mg of autologous immunoglobulin G (IgG) twice a week for 4 weeks (autologous immunoglobulin therapy, AIGT) and followed up for more than 2 years after the treatment. We observed the following 4 major findings in these 3 patients during the long-term follow-up after AIGT. (1) Two of the 3 patients showed a long-term clinical improvement for more than 36 weeks after AIGT with a maximum decrease in clinical severity score greater than 80% from baseline. (2) These 2 patients also showed long-term decreases in serum total IgE concentrations and peripheral blood eosinophil count for more than 36 weeks after AIGT with a maximum decrease in the two laboratory parameters of allergic inflammatory greater than 70% from baseline. (3) No significant side effect was observed during the 2 years of follow-up period after the AIGT in all 3 patients. (4) Serum levels of IgG anti-idiotype antibodies to the F(ab')2 fragment of autologous IgG administered for the treatment were not significantly changed after AIGT in all 3 patients. These findings suggest that AIGT has long-term favorable effects on both clinical severity and laboratory parameters in selected patients with severe recalcitrant AD. Further studies are required to evaluate the clinical usefulness and therapeutic mechanism of AIGT for AD.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Clinical Efficacy of Subcutaneous Allergen Immunotherapy in Patients with Atopic Dermatitis
Dong-Ho Nahm, Myoung-Eun Kim, Byul Kwon, Su-Mi Cho, Areum Ahn
Yonsei Med J. 2016;57(6):1420-1426. doi: 10.3349/ymj.2016.57.6.1420.
Reference
-
1. Nahm DH. Personalized immunomodulatory therapy for atopic dermatitis: an allergist's view. Ann Dermatol. 2015; 27:355–363.2. Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014; 371:130–139.3. Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB Jr, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005; 22:192–199.4. Schäfer T. Epidemiology of complementary alternative medicine for asthma and allergy in Europe and Germany. Ann Allergy Asthma Immunol. 2004; 93:S5–S10.5. Asefi M, Augustin M. Regulative therapy: treatment with nonspecific stimulants in dermatology in traditional and modern perspectives. Forsch Komplementarmed. 1999; 6:Suppl 2. 9–13.6. Pittler MH, Armstrong NC, Cox A, Collier PM, Hart A, Ernst E. Randomized, double-blind, placebo-controlled trial of autologous blood therapy for atopic dermatitis. Br J Dermatol. 2003; 148:307–313.7. Staubach P, Onnen K, Vonend A, Metz M, Siebenhaar F, Tschentscher I, et al. Autologous whole blood injections to patients with chronic urticaria and a positive autologous serum skin test: a placebo-controlled trial. Dermatology. 2006; 212:150–159.8. Debbarman P, Sil A, Datta PK, Bandyopadhyay D, Das NK. Autologous serum therapy in chronic urticaria: a promising complement to antihistamines. Indian J Dermatol. 2014; 59:375–382.9. Nahm DH, Cho SM, Kim ME, Kim YJ, Jeon SY. Autologous immunoglobulin therapy in patients with severe recalcitrant atopic dermatitis: a preliminary report. Allergy Asthma Immunol Res. 2014; 6:89–94.10. Nahm DH, Kim ME, Cho SM. Effects of intramuscular injection of autologous immunoglobulin on clinical severity and serum IgE concentration in patients with atopic dermatitis. Dermatology. 2015; 231:145–151.11. Hanifin JM, Rajka G. Diagnostic features of atopic eczema. Acta Derm Venereol Suppl (Stockh). 1980; 92:S44–S47.12. Stalder JF, Taïeb A. European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993; 186:23–31.13. Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007; 157:645–648.14. Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013; 131:1288–1296.e3.15. Valenta R, Campana R, Marth K, van Hage M. Allergen-specific immunotherapy: from therapeutic vaccines to prophylactic approaches. J Intern Med. 2012; 272:144–157.16. Lee J, Park CO, Lee KH. Specific immunotherapy in atopic dermatitis. Allergy Asthma Immunol Res. 2015; 7:221–229.17. Tuft L. Studies in atopic dermatitis. V. Problems in inhalant hyposensitization and results of treatment. J Allergy. 1960; 31:1–11.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autologous Immunoglobulin Therapy in Patients With Severe Recalcitrant Atopic Dermatitis: A Preliminary Report
- A Study on the Relationship of the Severity of Atopic Dermatitis, Serum IgE and IFN-gamma
- A Study on the Cell - Mediated Immunity of Patients with Apopic Dermatitis
- A Comparison of Two Scoring Methods in Atopic Dermatitis
- The efficacy of thymopentin in the treatment of atopic dermatitis