Ann Rehabil Med.
2014 Aug;38(4):494-505. 10.5535/arm.2014.38.4.494.
Evaluating the Differential Electrophysiological Effects of the Focal Vibrator on the Tendon and Muscle Belly in Healthy People
- Affiliations
-
- 1Department of Rehabilitation Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. keepwiz@naver.com
- 2Center for Bionics, Biomedical Research Institute, Korea Institute of Science and Technology, Seoul, Korea.
- KMID: 2165732
- DOI: http://doi.org/10.5535/arm.2014.38.4.494
Abstract
OBJECTIVE
To investigate the electrophysiological effects of focal vibration on the tendon and muscle belly in healthy people.
METHODS
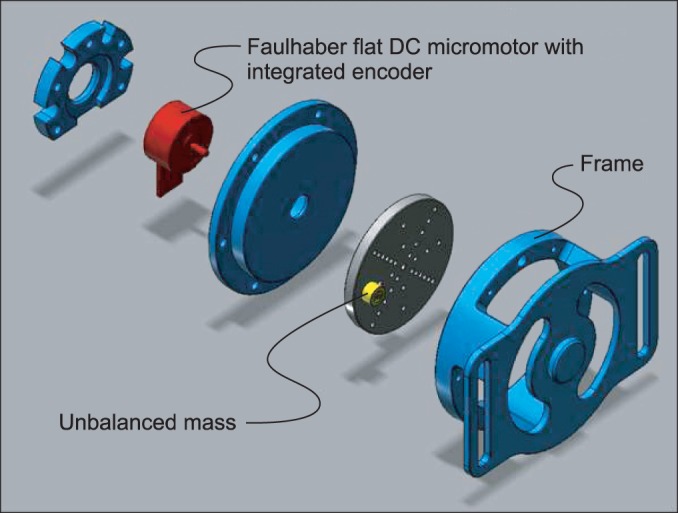
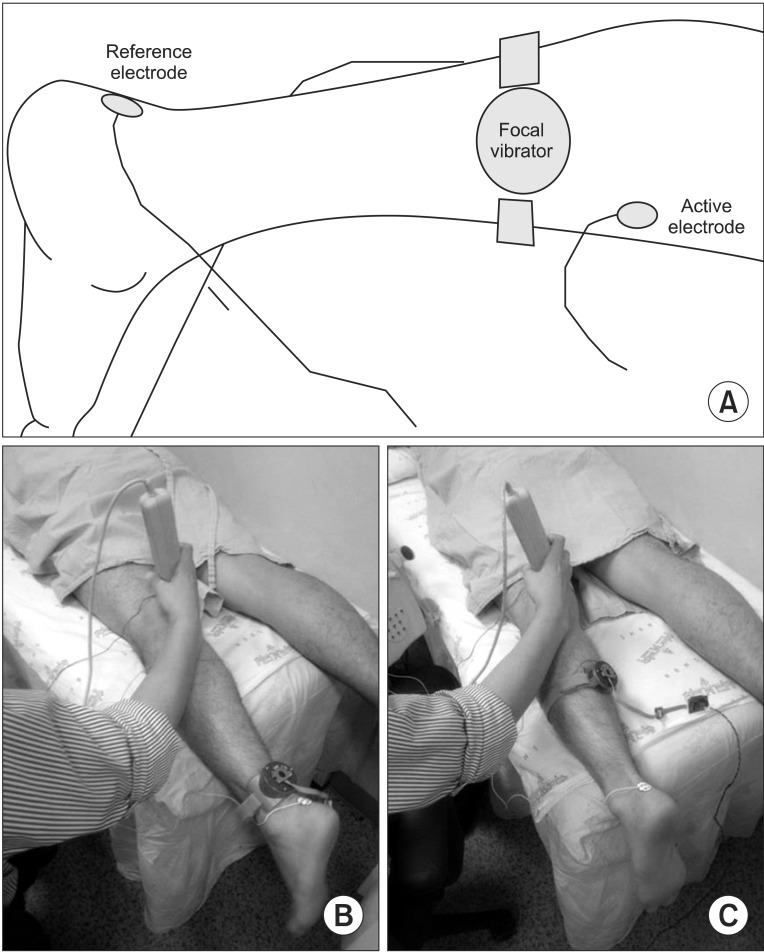
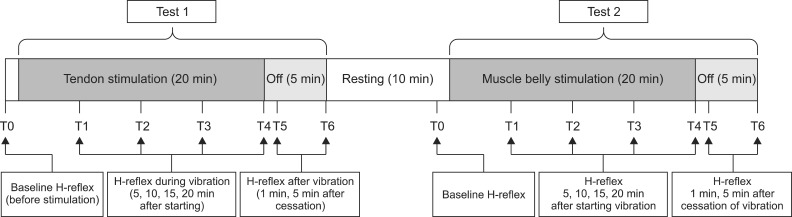
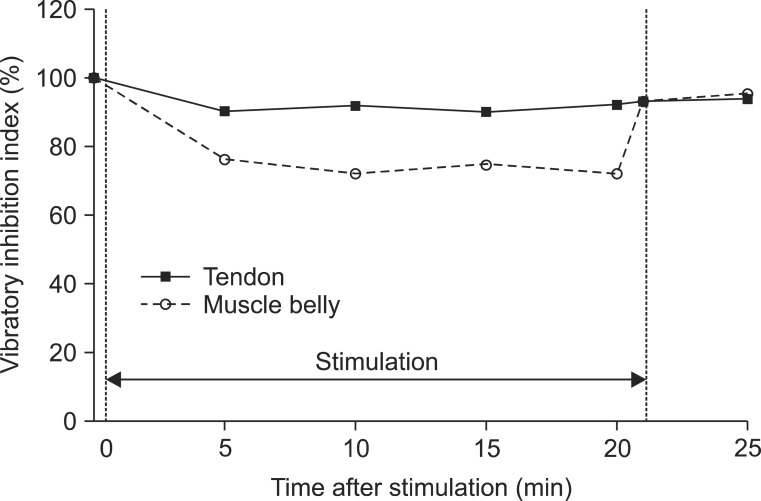
The miniaturized focal vibrator consisted of an unbalanced mass rotating offset and wireless controller. The parameters of vibratory stimulation were adjusted on a flat rigid surface as 65 microm at 70 Hz. Two consecutive tests on the different vibration sites were conducted in 10 healthy volunteers (test 1, the Achilles tendon; test 2, the muscle belly on the medial head of the gastrocnemius). The Hoffman (H)-reflex was measured 7 times during each test. The minimal H-reflex latency, maximal amplitude of H-reflex (Hmax), and maximal amplitude of the M-response (Mmax) were acquired. The ratio of Hmax and Mmax (HMR) and the vibratory inhibition index (VII: the ratio of the Hmax after vibration and Hmax before vibration) were calculated. The changes in parameters according to the time and site of stimulation were analyzed using the generalized estimating equation methods.
RESULTS
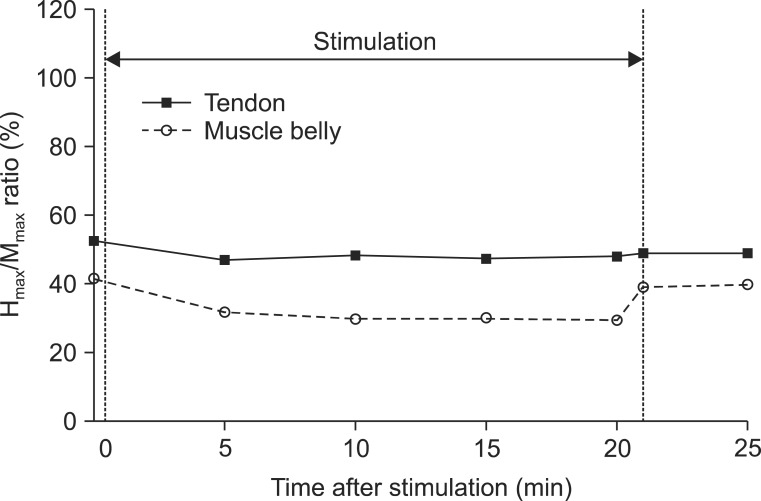
All subjects completed the two tests without serious adverse effects. The minimal H-reflex latency did not show significant changes over time (Wald test: chi2=11.62, p=0.07), and between the two sites (chi2=0.42, p=0.52). The changes in Hmax (chi2=53.74, p<0.01), HMR (chi2=20.49, p<0.01), and VII (chi2=13.16, p=0.02) were significant over time with the adjustment of sites. These parameters were reduced at all time points compared to the baseline, but the decrements reverted instantly after the cessation of stimulation. When adjusted over time, a 1.99-mV decrease in the Hmax (chi2=4.02, p=0.04) and a 9.02% decrease in the VII (chi2=4.54, p=0.03) were observed when the muscle belly was vibrated compared to the tendon.
CONCLUSION
The differential electrophysiological effects of focal vibration were verified. The muscle belly may be the more effective site for reducing the H-reflex compared to the tendon. This study provides the neurophysiological basis for a selective and safe rehabilitation program for spasticity management with focal vibration.
MeSH Terms
Figure
Reference
-
1. Wissel J, Manack A, Brainin M. Toward an epidemiology of poststroke spasticity. Neurology. 2013; 80(3 Suppl 2):S13–S19. PMID: 23319481.
Article2. Zorowitz RD, Gillard PJ, Brainin M. Poststroke spasticity: sequelae and burden on stroke survivors and caregivers. Neurology. 2013; 80(3 Suppl 2):S45–S52. PMID: 23319485.
Article3. Sunnerhagen KS, Olver J, Francisco GE. Assessing and treating functional impairment in poststroke spasticity. Neurology. 2013; 80(3 Suppl 2):S35–S44. PMID: 23319484.4. Francisco GE, McGuire JR. Poststroke spasticity management. Stroke. 2012; 43:3132–3136. PMID: 22984012.
Article5. Ness LL, Field-Fote EC. Effect of whole-body vibration on quadriceps spasticity in individuals with spastic hypertonia due to spinal cord injury. Restor Neurol Neurosci. 2009; 27:621–631. PMID: 20042786.
Article6. Ahlborg L, Andersson C, Julin P. Whole-body vibration training compared with resistance training: effect on spasticity, muscle strength and motor performance in adults with cerebral palsy. J Rehabil Med. 2006; 38:302–308. PMID: 16931460.
Article7. Lee BK, Chon SC. Effect of whole body vibration training on mobility in children with cerebral palsy: a randomized controlled experimenter-blinded study. Clin Rehabil. 2013; 27:599–607. PMID: 23411791.
Article8. Lau RW, Yip SP, Pang MY. Whole-body vibration has no effect on neuromotor function and falls in chronic stroke. Med Sci Sports Exerc. 2012; 44:1409–1418. PMID: 22330025.
Article9. Brogardh C, Flansbjer UB, Lexell J. No specific effect of whole-body vibration training in chronic stroke: a double-blind randomized controlled study. Arch Phys Med Rehabil. 2012; 93:253–258. PMID: 22289234.10. Conrad MO, Scheidt RA, Schmit BD. Effects of wrist tendon vibration on targeted upper-arm movements in poststroke hemiparesis. Neurorehabil Neural Repair. 2011; 25:61–70. PMID: 20921324.
Article11. Paoloni M, Mangone M, Scettri P, Procaccianti R, Cometa A, Santilli V. Segmental muscle vibration improves walking in chronic stroke patients with foot drop: a randomized controlled trial. Neurorehabil Neural Repair. 2010; 24:254–262. PMID: 19855076.
Article12. Noma T, Matsumoto S, Etoh S, Shimodozono M, Kawahira K. Anti-spastic effects of the direct application of vibratory stimuli to the spastic muscles of hemiplegic limbs in post-stroke patients. Brain Inj. 2009; 23:623–631. PMID: 19557565.
Article13. Marconi B, Filippi GM, Koch G, Giacobbe V, Pecchioli C, Versace V, et al. Long-term effects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehabil Neural Repair. 2011; 25:48–60. PMID: 20834043.
Article14. Liepert J, Binder C. Vibration-induced effects in stroke patients with spastic hemiparesis: a pilot study. Restor Neurol Neurosci. 2010; 28:729–735. PMID: 21209488.15. Suresh NL, Wang I, Heckman CJ, Rymer WZ. Characterization of the tendon vibration reflex response in hemi-spastic stroke individuals. Conf Proc IEEE Eng Med Biol Soc. 2011; 2011:2053–2056. PMID: 22254740.
Article16. Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2012; 92:1651–1697. PMID: 23073629.
Article17. Van Boxtel A. Differential effects of low-frequency depression, vibration-induced inhibition, and posttetanic potentiation on H-reflexes and tendon jerks in the human soleus muscle. J Neurophysiol. 1986; 55:551–568. PMID: 3514814.
Article18. Iles JF, Roberts RC. Inhibition of monosynaptic reflexes in the human lower limb. J Physiol. 1987; 385:69–87. PMID: 2958622.
Article19. Noma T, Matsumoto S, Shimodozono M, Etoh S, Kawahira K. Anti-spastic effects of the direct application of vibratory stimuli to the spastic muscles of hemiplegic limbs in post-stroke patients: a proof-of-principle study. J Rehabil Med. 2012; 44:325–330. PMID: 22402727.
Article20. Marconi B, Filippi GM, Koch G, Pecchioli C, Salerno S, Don R, et al. Long-term effects on motor cortical excitability induced by repeated muscle vibration during contraction in healthy subjects. J Neurol Sci. 2008; 275:51–59. PMID: 18760809.
Article21. Caliandro P, Celletti C, Padua L, Minciotti I, Russo G, Granata G, et al. Focal muscle vibration in the treatment of upper limb spasticity: a pilot randomized controlled trial in patients with chronic stroke. Arch Phys Med Rehabil. 2012; 93:1656–1661. PMID: 22507444.
Article22. Fallon JB, Macefield VG. Vibration sensitivity of human muscle spindles and Golgi tendon organs. Muscle Nerve. 2007; 36:21–29. PMID: 17471568.
Article23. Kokkorogiannis T. Somatic and intramuscular distribution of muscle spindles and their relation to muscular angiotypes. J Theor Biol. 2004; 229:263–280. PMID: 15207480.
Article24. Jozsa L, Balint J, Kannus P, Jarvinen M, Lehto M. Mechanoreceptors in human myotendinous junction. Muscle Nerve. 1993; 16:453–457. PMID: 8515753.
Article25. Banks RW, Hulliger M, Saed HH, Stacey MJ. A comparative analysis of the encapsulated end-organs of mammalian skeletal muscles and of their sensory nerve endings. J Anat. 2009; 214:859–887. PMID: 19538631.
Article26. Somerville J, Ashby P. Hemiplegic spasticity: neurophysiologic studies. Arch Phys Med Rehabil. 1978; 59:592–596. PMID: 736764.27. Voerman GE, Gregoric M, Hermens HJ. Neurophysiological methods for the assessment of spasticity: the Hoffmann reflex, the tendon reflex, and the stretch reflex. Disabil Rehabil. 2005; 27:33–68. PMID: 15799143.
Article28. Desmedt JE, Godaux E. Mechanism of the vibration paradox: excitatory and inhibitory effects of tendon vibration on single soleus muscle motor units in man. J Physiol. 1978; 285:197–207. PMID: 154563.
Article29. Lance JW. Disordered muscle tone and movement. Clin Exp Neurol. 1981; 18:27–35. PMID: 6926389.30. Burke D, Wissel J, Donnan GA. Pathophysiology of spasticity in stroke. Neurology. 2013; 80(3 Suppl 2):S20–S26. PMID: 23319482.
Article31. Misiaszek JE. The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve. 2003; 28:144–160. PMID: 12872318.
Article32. Earles DR, Morris HH, Peng CY, Koceja DM. Assessment of motoneuron excitability using recurrent inhibition and paired reflex depression protocols: a test of reliability. Electromyogr Clin Neurophysiol. 2002; 42:159–166. PMID: 11977429.33. Burke D, Ashby P. Are spinal "presynaptic" inhibitory mechanisms suppressed in spasticity? J Neurol Sci. 1972; 15:321–326. PMID: 5014095.
Article34. Ali AA, Sabbahi MA. H-reflex changes under spinal loading and unloading conditions in normal subjects. Clin Neurophysiol. 2000; 111:664–670. PMID: 10727917.
Article35. Levin MF, Hui-Chan C. Are H and stretch reflexes in hemiparesis reproducible and correlated with spasticity? J Neurol. 1993; 240:63–71. PMID: 8437021.
Article36. Brouwer B, de Andrade VS. The effects of slow stroking on spasticity in patients with multiple sclerosis: a pilot study. Physiother Theory Pract. 1995; 11:13–21.
Article37. Fourment A, Chennevelle JM, Belhaj-Saif A, Maton B. Responses of motor cortical cells to short trains of vibration. Exp Brain Res. 1996; 111:208–214. PMID: 8891651.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Separated muscle belly of the flexor digitorum brevis for the fifth toe: a case report
- A clinical perspective on the anatomical study of digastric muscle
- Bicipital origin and the course of the plantaris muscle
- Unusual morphology of the superior belly of omohyoid muscle
- The Co-existence of the Gastrocnemius Tertius and Accessory Soleus Muscles