Current Guidelines in the Management of Upper Gastrointestinal Subepithelial Tumors
- Affiliations
-
- 1Department of Internal Medicine, Presbyterian Medical Center, Jeonju, Korea. jeja-1004@hanmail.net
- KMID: 2165018
- DOI: http://doi.org/10.5946/ce.2015.096
Abstract
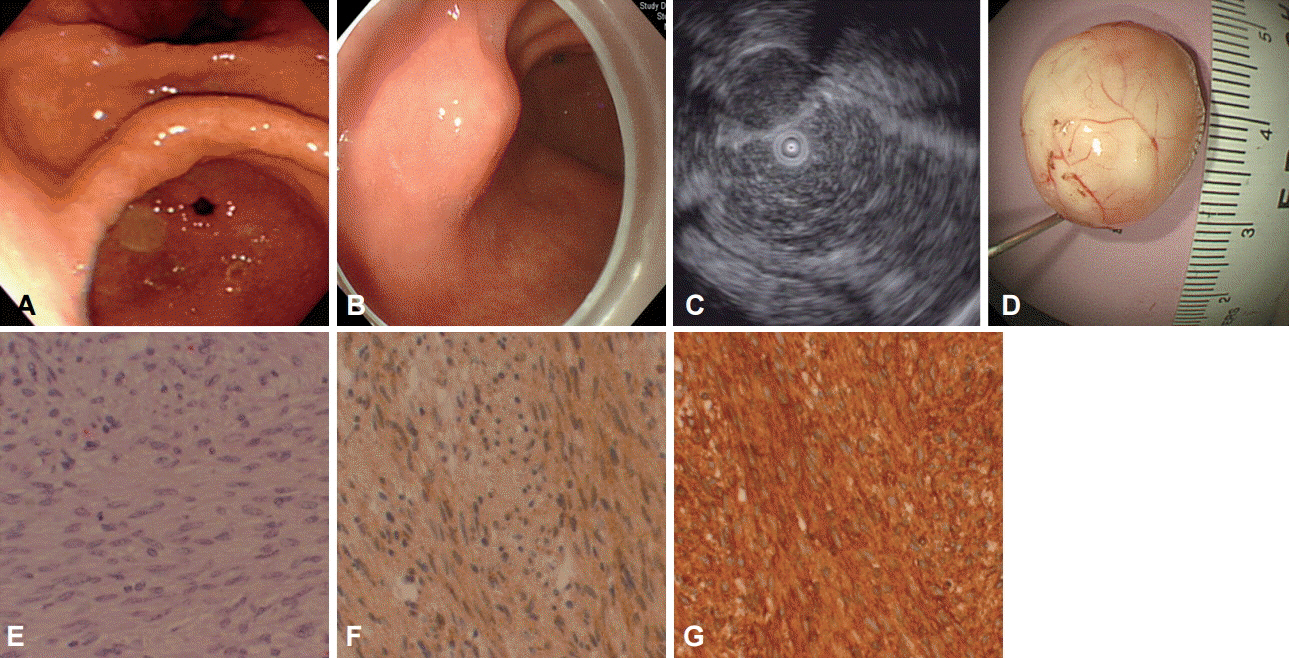
- Subepithelial tumors are frequently found in asymptomatic patients in Japan and Korea where cancer screening tests routinely include endoscopy. Most lesions are asymptomatic and clinically insignificant. However, carcinoid tumors, lymphomas, glomus tumor and gastrointestinal stromal tumors (GISTs) are malignant or have the potential to become malignant. Inflammation due to parasitic infestation by Anisakis and poorly differentiated adenocarcinomas in the stomach rarely present as subepithelial lesions. In contrast to the frequency of gastric GIST in the gastrointestinal system, they are uncommon in the duodenum and very rare in the esophagus. The prognosis of patients with GISTs in the stomach is relatively good compared with GISTs in other organs. Along with the location of the tumor, its size and mitotic count are major factors that determine the malignant potential of GIST. Small (<2 cm) asymptomatic GISTs usually have benign clinical course. GIST is the most common subepithelial tumor to occur in the stomach. Although various methods are employed to diagnose GISTs, the risk of GIST metastasis cannot be accurately predicted before lesions are completely resected. Recently, new endoscopic diagnostic methods and treatment techniques have been developed that allow the diagnosis and resection of lesions located in the muscularis propria, without any complications. These endoscopic methods have different indications depending on regions where they are performed.
MeSH Terms
Figure
Cited by 9 articles
-
Predictive Factors for Differentiating Gastrointestinal Stromal Tumors from Leiomyomas Based on Endoscopic Ultrasonography Findings in Patients with Gastric Subepithelial Tumors: A Multicenter Retrospective Study
Sun Moon Kim, Eun Young Kim, Jin Woong Cho, Seong Woo Jeon, Ji Hyun Kim, Tae Hyeon Kim, Jeong Seop Moon, Jin-Oh Kim
Clin Endosc. 2021;54(6):872-880. doi: 10.5946/ce.2021.251.Endoscopic Ultrasound-Guided Fine Needle Aspiration and Biopsy in Gastrointestinal Subepithelial Tumors
Gyu Young Pih, Do Hoon Kim
Clin Endosc. 2019;52(4):314-320. doi: 10.5946/ce.2019.100.Diagnosis of Gastric Subepithelial Tumors Using Endoscopic Ultrasonography or Abdominopelvic Computed Tomography: Which is Better?
Eun Young Park, Gwang Ha Kim
Clin Endosc. 2019;52(6):519-520. doi: 10.5946/ce.2019.188.Endoscopic Ultrasonography in the Diagnosis of Gastric Subepithelial Lesions
Eun Jeong Gong, Do Hoon Kim
Clin Endosc. 2016;49(5):425-433. doi: 10.5946/ce.2016.065.Is Endoscopic Ultrasonography Adequate for the Diagnosis of Gastric Schwannomas?
Eun Jeong Gong, Kee Don Choi
Clin Endosc. 2016;49(6):498-499. doi: 10.5946/ce.2016.134.Prevalence, natural progression, and clinical practices of upper gastrointestinal subepithelial lesions in Korea: a multicenter study
Younghee Choe, Yu Kyung Cho, Gwang Ha Kim, Jun-Ho Choi, Eun Soo Kim, Ji Hyun Kim, Eun Kwang Choi, Tae Hyeon Kim, Seong-Hun Kim, Do Hoon Kim
Clin Endosc. 2023;56(6):744-753. doi: 10.5946/ce.2023.005.Endoscopic resection of gastric gastrointestinal stromal tumor using clip-and-cut endoscopic full-thickness resection: a single-center, retrospective cohort in Korea
Yuri Kim, Ji Yong Ahn, Hwoon-Yong Jung, Seokin Kang, Ho June Song, Kee Don Choi, Do Hoon Kim, Jeong Hoon Lee, Hee Kyong Na, Young Soo Park
Clin Endosc. 2024;57(3):350-363. doi: 10.5946/ce.2023.144.Endoscopic resection penetrating the muscularis propria for gastric gastrointestinal stromal tumors: advances and challenges
Jin Woong Cho
Clin Endosc. 2024;57(3):329-331. doi: 10.5946/ce.2024.036.Prevalence and natural course of incidental gastric subepithelial tumors
Dae-Hyuk Heo, Min A Yang, Jae Sun Song, Won Dong Lee, Jin Woong Cho
Clin Endosc. 2024;57(4):495-500. doi: 10.5946/ce.2023.124.
Reference
-
1. Lim YJ, Son HJ, Lee JS, et al. Clinical course of subepithelial lesions detected on upper gastrointestinal endoscopy. World J Gastroenterol. 2010; 16:439–444.
Article2. Gill KR, Camellini L, Conigliaro R, et al. The natural history of upper gastrointestinal subepithelial tumors: a multicenter endoscopic ultrasound survey. J Clin Gastroenterol. 2009; 43:723–726.3. Kim MY, Jung HY, Choi KD, et al. Natural history of asymptomatic small gastric subepithelial tumors. J Clin Gastroenterol. 2011; 45:330–336.
Article4. Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006; 37:1527–1535.
Article5. Agaimy A, Wünsch PH, Dirnhofer S, Bihl MP, Terracciano LM, Tornillo L. Microscopic gastrointestinal stromal tumors in esophageal and intestinal surgical resection specimens: a clinicopathologic, immunohistochemical, and molecular study of 19 lesions. Am J Surg Pathol. 2008; 32:867–873.6. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006; 23:70–83.
Article7. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008; 39:1411–1419.
Article8. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005; 29:52–68.9. Tryggvason G, Gíslason HG, Magnússon MK, Jónasson JG. Gastrointestinal stromal tumors in Iceland, 1990-2003: the icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer. 2005; 117:289–293.
Article10. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002; 33:459–465.
Article11. Trupiano JK, Stewart RE, Misick C, Appelman HD, Goldblum JR. Gastric stromal tumors: a clinicopathologic study of 77 cases with correlation of features with nonaggressive and aggressive clinical behaviors. Am J Surg Pathol. 2002; 26:705–714.12. Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era: a population-based study in western Sweden. Cancer. 2005; 103:821–829.13. Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010; 8(Suppl 2):S1–S41.
Article14. Lachter J, Bishara N, Rahimi E, Shiller M, Cohen H, Reshef R. EUS clarifies the natural history and ideal management of GISTs. Hepatogastroenterology. 2008; 55:1653–1656.15. Khashab MA, Pasricha PJ. Conquering the third space: challenges and opportunities for diagnostic and therapeutic endoscopy. Gastrointest Endosc. 2013; 77:146–148.
Article16. Meesters B, Pauwels PA, Pijnenburg AM, Vlasveld LT, Repelaer van Driel OJ. Metastasis in a benign duodenal stromal tumour. Eur J Surg Oncol. 1998; 24:334–335.
Article17. Nishida T, Kawai N, Yamaguchi S, Nishida Y. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013; 25:479–489.
Article18. ASGE Standards of Practice Committee, Gan SI, Rajan E, et al. Role of EUS. Gastrointest Endosc. 2007; 66:425–434.
Article19. Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005; 62:202–208.
Article20. Karaca C, Turner BG, Cizginer S, Forcione D, Brugge W. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010; 71:722–727.
Article21. Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000; 46:88–92.
Article22. Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011; 43:897–912.
Article23. ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014; 25 Suppl 3:iii21–iii26.24. Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008; 13:416–430.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Techniques for Treating Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract
- Incidental Gastrointestinal Subepithelial Mass
- The Diagnosis of Subepithelial Lesions in the Upper Gastrointestinal Tract
- Endoscopic Treatment of Submucosal Tumor from Upper Gastrointestinal Tract
- Common Gastric Subepithelial Tumors in Koreans