J Korean Med Sci.
2015 Aug;30(8):1001-1009. 10.3346/jkms.2015.30.8.1001.
Treatment of Helicobacter pylori Infection in Korea: A Systematic Review and Meta-analysis
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea. jgkimd@cau.ac.kr
- 2Institute for Evidence-based Medicine, Department of Preventive Medicine, College of Medicine, Korea University, Seoul, Korea.
- KMID: 2164492
- DOI: http://doi.org/10.3346/jkms.2015.30.8.1001
Abstract
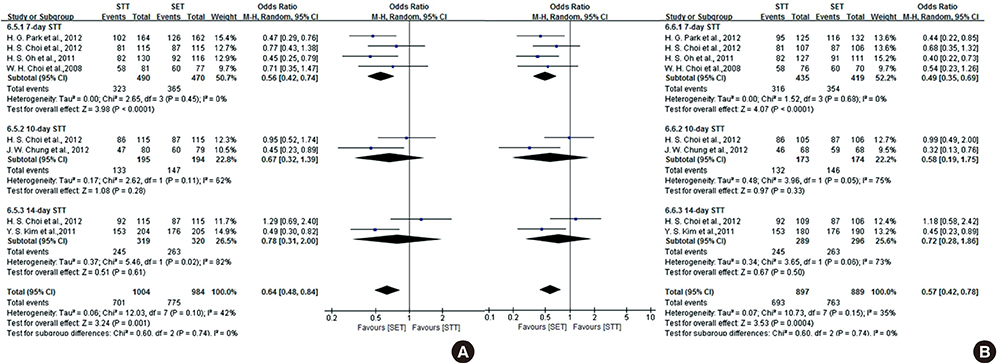
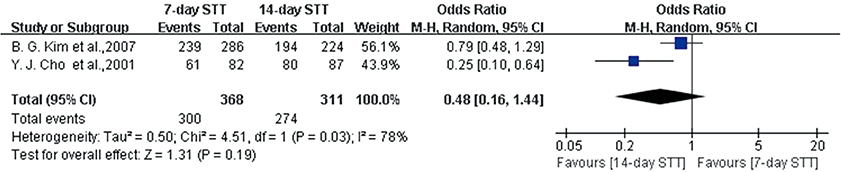
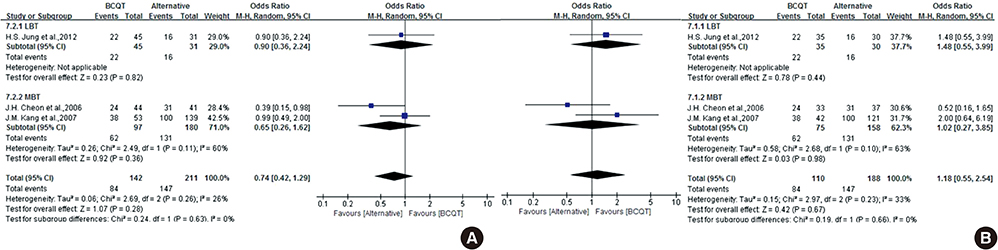
- The efficacy of seven-day clarithromycin-based standard triple therapy (STT) for Helicobacter pylori has decreased in Korea over the past decade. The aim of this meta-analysis was to clarify the efficacy of first-line and second-line therapies in Korea. This systematic review will provide an overview of H. pylori eradication and present new therapeutic strategies used in Korea. An extensive search of the literature concerning STT, sequential therapy (SET), concomitant therapy (CT), bismuth-containing quadruple therapy (BCQT) and various other therapies used in Korea was performed. All selected studies were randomized controlled trials (RCTs). Eighteen RCTs were eligible for systematic review. The alternative regimens comparing seven-day STT as a first-line therapy include SET, CT, levofloxacin-based therapy (LBT), BCQT, and STT with prolonged duration. The results of the meta-analysis suggest that SET is superior to seven-day STT. The overall eradication rate by intention to treat (ITT) analysis was 69.8% for STT and 79.7% for SET. The overall eradication rate by per-protocol (PP) analysis was 77.0% for STT and 85.0% for SET. The odds ratios for the ITT and PP eradication rate were 0.57 (95% confidence interval [CI], 0.43 to 0.74) and 0.52 (95% CI, 0.35 to 0.76), respectively. In the subgroup analysis, however, there were no significant differences between SET and STT with prolonged durations. Alternative regimens to seven-day BCQT as second-line therapy include LBT, moxifloxacin-based therapy and 14-day BCQT. The eradication rates of these alternative regimens were not superior to that of the conventional treatment. SET is superior to seven-day STT but not to STT with prolonged duration.
Keyword
MeSH Terms
-
Anti-Bacterial Agents/*administration & dosage/classification
Evidence-Based Medicine
Female
Helicobacter Infections/*drug therapy/*epidemiology/microbiology
Helicobacter pylori/*drug effects
Humans
Male
Practice Patterns, Physicians'/*statistics & numerical data
Prevalence
Republic of Korea/epidemiology
Risk Assessment
Treatment Outcome
Anti-Bacterial Agents
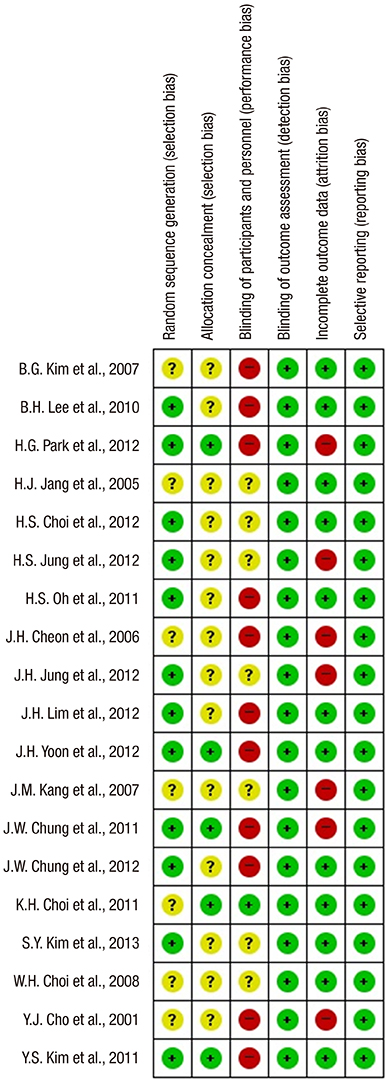
Figure
Reference
-
1. Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection: the Maastricht IV/ Florence Consensus Report. Gut. 2012; 61:646–664.2. Chung JW, Lee GH, Han JH, Jeong JY, Choi KS, Kim DH, Jung KW, Choi KD, Song HJ, Jung HY, et al. The trends of one-week first-line and second-line eradication therapy for Helicobacter pylori infection in Korea. Hepatogastroenterology. 2011; 58:246–250.3. Kim JS, Ji JS, Choi H, Kim JH. Sequential therapy or triple therapy for Helicobacter pylori infection in Asians: systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2014; 38:118–125.4. Yoon H, Lee DH, Kim N, Park YS, Shin CM, Kang KK, Oh DH, Jang DK, Chung JW. Meta-analysis: is sequential therapy superior to standard triple therapy for Helicobacter pylori infection in Asian adults? J Gastroenterol Hepatol. 2013; 28:1801–1809.5. Kim JS, Kim BW, Ham JH, Park HW, Kim YK, Lee MY, Ji JS, Lee BI, Choi H. Sequential therapy for Helicobacter pylori infection in Korea: systematic review and meta-analysis. Gut Liver. 2013; 7:546–551.6. Chung JW, Ha M, Yun SC, Kim JH, Lee JJ, Kim YJ, Kim KO, Kwon KA, Park DK, Lee DH. Meta-analysis: sequential therapy is superior to conventional therapy for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2013; 62:267–271.7. Jung HS, Shim KN, Baik SJ, Na YJ, Kang MJ, Jung JM, Ha CY, Jung SA, Yoo K. Efficacy of levofloxacin-based triple therapy as second-line Helicobacter pylori eradication. Korean J Gastroenterol. 2008; 51:285–290.8. Yoon H, Kim N, Lee BH, Hwang TJ, Lee DH, Park YS, Nam RH, Jung HC, Song IS. Moxifloxacin-containing triple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate. Helicobacter. 2009; 14:77–85.9. Chung JW, Jung YK, Kim YJ, Kwon KA, Kim JH, Lee JJ, Lee SM, Hahm KB, Lee SM, Jeong JY, et al. Ten-day sequential versus triple therapy for Helicobacter pylori eradication: a prospective, open-label, randomized trial. J Gastroenterol Hepatol. 2012; 27:1675–1680.10. Choi HS, Chun HJ, Park SH, Keum B, Seo YS, Kim YS, Jeen YT, Um SH, Lee HS, Kim CD, et al. Comparison of sequential and 7-, 10-, 14-d triple therapy for Helicobacter pylori infection. World J Gastroenterol. 2012; 18:2377–2382.11. Park HG, Jung MK, Jung JT, Kwon JG, Kim EY, Seo HE, Lee JH, Yang CH, Kim ES, Cho KB, et al. Randomised clinical trial: a comparative study of 10-day sequential therapy with 7-day standard triple therapy for Helicobacter pylori infection in naive patients. Aliment Pharmacol Ther. 2012; 35:56–65.12. Kim YS, Kim SJ, Yoon JH, Suk KT, Kim JB, Kim DJ, Kim DY, Min HJ, Park SH, Shin WG, et al. Randomised clinical trial: the efficacy of a 10-day sequential therapy vs. a 14-day standard proton pump inhibitor-based triple therapy for Helicobacter pylori in Korea. Aliment Pharmacol Ther. 2011; 34:1098–1105.13. Oh HS, Lee DH, Seo JY, Cho YR, Kim N, Jeoung SH, Kim JW, Hwang JH, Park YS, Lee SH, et al. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: a prospective, randomized study. J Gastroenterol Hepatol. 2012; 27:504–509.14. Choi WH, Park DI, Oh SJ, Baek YH, Hong CH, Hong EJ, Song MJ, Park SK, Park JH, Kim HJ, et al. Effectiveness of 10 day-sequential therapy for Helicobacter pylori eradication in Korea. Korean J Gastroenterol. 2008; 51:280–284.15. Kim SY, Lee SW, Hyun JJ, Jung SW, Koo JS, Yim HJ, Park JJ, Chun HJ, Choi JH. Comparative study of Helicobacter pylori eradication rates with 5-day quadruple "concomitant" therapy and 7-day standard triple therapy. J Clin Gastroenterol. 2013; 47:21–24.16. Lim JH, Lee DH, Choi C, Lee ST, Kim N, Park YS, Shin CM, Cho HJ. Two weeks sequential and concomitant therapy for Helicobacter pylori infection and factors associated with eradication: a prospective randomized trial. Journal of Gastroenterology and Hepatology; 111 River ST, Hoboken 07030-5774. NJ USA: Wiley-Balckwell;2012. p. 60.17. Jang HJ, Choi MH, Kim YS, Seo YA, Baik KH, Baik IH, Eun CS, Kim JB, Kae SH, Kim DJ, et al. Effectiveness of triple therapy and quadruple therapy for Helicobacter pylori eradication. Korean J Gastroenterol. 2005; 46:368–372.18. Choi KH, Chung WC, Lee KM, Paik CN, Kim EJ, Kang BK, Oak JH, Jung SH. Efficacy of levofloxacin and rifaximin based quadruple therapy in Helicobacter pylori associated gastroduodenal disease: a double-blind, randomized controlled trial. J Korean Med Sci. 2011; 26:785–790.19. Cho YJ, Chun HJ, Kim ST, Koh DW, Park JH, Park DK, Park CH, Lee SJ, Jeen YT, Lee HS, et al. Analysis of eradication rate of Helicobacter pylori according to treatment duration by using 13C-urea breath test comparison of OAC 7, 10 or 14 days regimen. Korean J Gastrointest Endosc. 2001; 23:207–212.20. Kim BG, Lee DH, Ye BD, Lee KH, Kim BW, Kim SG, Kim SW, Kim SK, Kim JJ, Kim HY, et al. Comparison of 7-day and 14-day proton pump inhibitor-containing triple therapy for Helicobacter pylori eradication: Neither treatment duration provides acceptable eradication rate in Korea. Helicobacter. 2007; 12:31–35.21. Cheon JH, Kim N, Lee DH, Kim JM, Kim JS, Jung HC, Song IS. Efficacy of moxifloxacin-based triple therapy as second-line treatment for Helicobacter pylori infection. Helicobacter. 2006; 11:46–51.22. Kang JM, Kim N, Lee DH, Park YS, Kim YR, Kim JS, Jung HC, Song IS. Second-line treatment for Helicobacter pylori infection: 10-Day moxifloxacin-based triple therapy versus 2-week quadruple therapy. Helicobacter. 2007; 12:623–628.23. Lee BH, Kim N, Hwang TJ, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Jung HC, et al. Bismuth-containing quadruple therapy as second-line treatment for Helicobacter pylori infection: Effect of treatment duration and antibiotic resistance on the eradication rate in Korea. Helicobacter. 2010; 15:38–45.24. Yoon JH, Baik GH, Kim YS, Suk KT, Shin WG, Kim KH, Kim KO, Park CH, Baik IH, Jang HJ, et al. Comparison of the eradication rate between 1- and 2-week bismuth-containing quadruple rescue therapies for Helicobacter pylori eradication. Gut Liver. 2012; 6:434–439.25. Chung JW, Lee JH, Jung HY, Yun SC, Oh TH, Choi KD, Song HJ, Lee GH, Kim JH. Second-line Helicobacter pylori eradication: a randomized comparison of 1-week or 2-week bismuth-containing quadruple therapy. Helicobacter. 2011; 16:289–294.26. Jeong MH, Chung JW, Lee SJ, Ha M, Jeong SH, Na S, Na BS, Park SK, Kim YJ, Kwon KA, et al. Comparison of rifabutin- and levofloxacin-based third-line rescue therapies for Helicobacter pylori. Korean J Gastroenterol. 2012; 59:401–406.27. Lee SK, Lee SW, Park JY, Kwon BS, Kim SY, Hyun JJ, Kim JH, Jung SW, Koo JS, Yim HJ, et al. Effectiveness and safety of repeated quadruple therapy in Helicobacter pylori infection after failure of second-line quadruple therapy: repeated quadruple therapy as a third-line therapy. Helicobacter. 2011; 16:410–414.28. Park HK, Lee DH, Suh S, Seo PJ, Kim N, Jeong SH, Kim JW, Hwang JH, Park YS, Lee SH, et al. Dual therapy trial using esomeprazole and amoxicillin as third-line rescue therapy for Helicobacter pylori infection. Clin Endosc. 2011; 44:33–37.29. Yun SP, Seon HG, Ok CS, Yoo KH, Kang MK, Kim WH, Kwon CI, Ko KH, Hwang SG, Park PW, et al. Rifaximin plus levofloxacin-based rescue regimen for the eradication of Helicobacter pylori. Gut Liver. 2012; 6:452–456.30. Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS. Korean College of Helicobacter and Upper Gastrointestinal Research. Korean Association of Gastroenterology. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009; 54:269–278.31. Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013; 347:f4587.32. Yuan Y, Ford AC, Khan KJ, Gisbert JP, Forman D, Leontiadis GI, Tse F, Calvet X, Fallone C, Fischbach L, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013; 12:CD008337.33. Gisbert JP, Calvet X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin Exp Gastroenterol. 2012; 5:23–34.34. Wang B, Wang YH, Lv ZF, Xiong HF, Wang H, Yang Y, Xie Y. Review: efficacy and safety of hybrid therapy for Helicobacter pylori infection: a systematic review and meta-analysis. Helicobacter. 2015; 20:79–88.35. Kim JM, Kim JS, Kim N, Kim SG, Jung HC, Song IS. Comparison of primary and secondary antimicrobial minimum inhibitory concentrations for Helicobacter pylori isolated from Korean patients. Int J Antimicrob Agents. 2006; 28:6–13.36. Xiao SP, Gu M, Zhang GX. Is levofloxacin-based triple therapy an alternative for first-line eradication of Helicobacter pylori? A systematic review and meta-analysis. Scand J Gastroenterol. 2014; 49:528–538.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Helicobacter pylori infection in functional dyspepsia
- Helicobacter pylori Eradication and Risks of Metachronous Recurrence after Endoscopic Resection of Gastric Adenoma: A Systematic Review and Meta-Analysis
- The Association between Helicobacter pylori Infection and Gastric Adenocarcinoma: a Review of the Literature
- Treatment of Helicobacter pylori infection
- Helicobacter pylori Infection in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis