Yonsei Med J.
2015 Sep;56(5):1266-1273. 10.3349/ymj.2015.56.5.1266.
Comparison of Second- and Third-Generation Cephalosporin as Initial Therapy for Women with Community-Onset Uncomplicated Acute Pyelonephritis
- Affiliations
-
- 1Department of Internal Medicine, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. wiesh@chol.com
- KMID: 2163618
- DOI: http://doi.org/10.3349/ymj.2015.56.5.1266
Abstract
- PURPOSE
This study examined the clinical effectiveness of parenteral cefuroxime and cefotaxime as empirical antibiotics for treating hospitalized women with uncomplicated acute pyelonephritis (APN).
MATERIALS AND METHODS
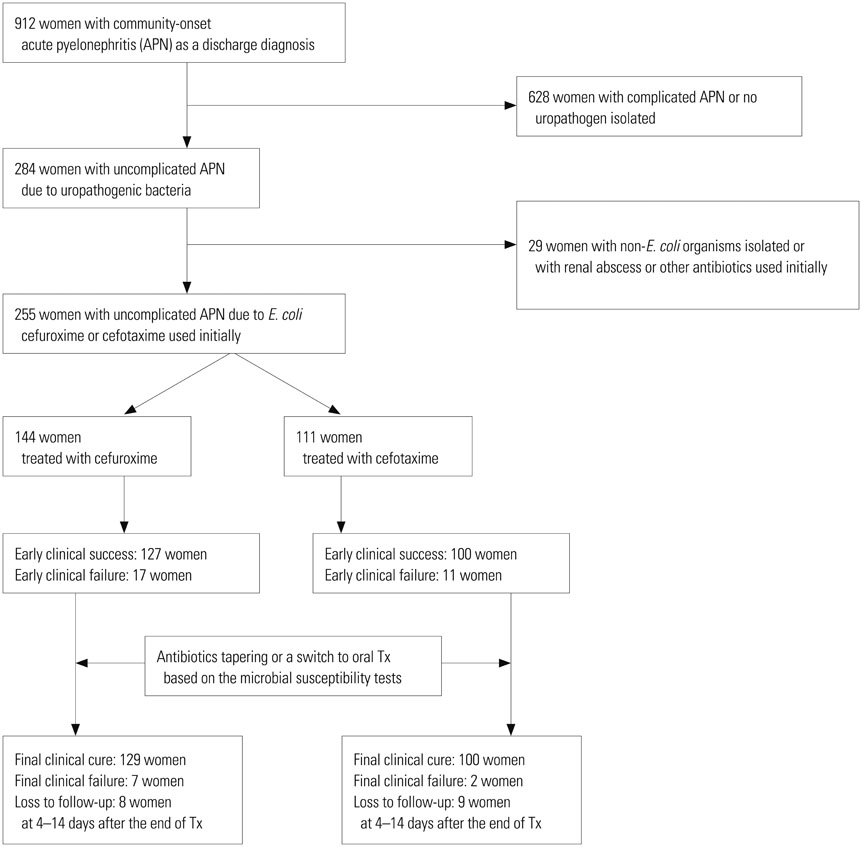
This study was based on the clinical and microbiologic data of 255 hospitalized women with APN. Of these 255 women, 144 patients received cefuroxime and 111 received cefotaxime.
RESULTS
There were no marked differences in the demographic features, clinical characteristics, and treatment duration between the populations of the cefuroxime and cefotaxime groups. The rates of defervescence showed no significant differences in the two groups at 48, 72, 96, and 120 hours. The clinical cure rates observed at the follow-up visit 4 to 14 days after the completion of antimicrobial therapy were not statistically different between the cefuroxime and cefotaxime groups [94.9% (129 of 136) versus 98.0% (100 of 102), respectively; p=0.307], and the microbiological cure rates were also not significantly different [88.3% (91 of 103) versus 95.0% (76 of 80), respectively; p=0.186]. The median hospitalization periods in the cefuroxime and cefotaxime groups were 7 (6-8) and 7 (6-8) days (p=0.157), respectively. Microbiological success rates after 72-96 hours of initial antimicrobial therapy were also not statistically different in the cefuroxime and cefotaxime groups, 89.4% (110 of 123) versus 94.9% (93 of 98; p=0.140).
CONCLUSION
Cefuroxime, a second-generation cephalosporin, is an appropriate antibiotic option for the initial treatment of uncomplicated APN and its efficacy does not differ from cefotaxime, a third-generation cephalosporin, in the initial parenteral therapy for community-onset APN.
Keyword
MeSH Terms
-
Administration, Intravenous
Adult
Aged
Anti-Bacterial Agents/administration & dosage/*therapeutic use
Cefotaxime/administration & dosage/*therapeutic use
Cefuroxime/administration & dosage/*therapeutic use
Community-Acquired Infections/*drug therapy
Escherichia coli/drug effects
Female
Humans
Infusions, Parenteral
Length of Stay
Male
Middle Aged
Pyelonephritis/*drug therapy/microbiology
Retrospective Studies
Treatment Outcome
Anti-Bacterial Agents
Cefotaxime
Cefuroxime
Figure
Cited by 1 articles
-
Clinical Practice Guidelines for the Antibiotic Treatment of Community-Acquired Urinary Tract Infections
Cheol-In Kang, Jieun Kim, Dae Won Park, Baek-Nam Kim, U-Syn Ha, Seung-Ju Lee, Jeong Kyun Yeo, Seung Ki Min, Heeyoung Lee, Seong-Heon Wie
Infect Chemother. 2018;50(1):67-100. doi: 10.3947/ic.2018.50.1.67.
Reference
-
1. Leigh DA, Joy GE, Tait S, Harris K, Walsh B. Treatment of acute uncomplicated urinary tract infections with single daily doses of cefuroxime axetil. J Antimicrob Chemother. 1989; 23:267–273.
Article2. O'Callaghan CH, Sykes RB, Griffiths A, Thornton JE. Cefuroxime, a new cephalosporin antibiotic: activity in vitro. Antimicrob Agents Chemother. 1976; 9:511–519.3. Jones RN, Fuchs PC, Gavan TL, Gerlach EH, Barry AL, Thornsberry C. Cefuroxime, a new parenteral cephalosporin: collaborative in vitro susceptibility comparison with cephalothin against 5,887 clinical bacterial isolates. Antimicrob Agents Chemother. 1977; 12:47–50.
Article4. Roberts AP, Phillips R, Griffiths A, Sykes RB. The sensitivity of urinary isolates to cefuroxime, a new beta-lactamase stable cephalosporin. Curr Med Res Opin. 1980; 7:5–13.
Article5. Nicolle LE. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol Clin North Am. 2008; 35:1–12.
Article6. Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011; 52:e103–e120.
Article7. Rodriguez-Baño J, Paterson DL. A change in the epidemiology of infections due to extended-spectrum beta-lactamase-producing organisms. Clin Infect Dis. 2006; 42:935–937.8. Wie SH, Kim HW, Chang UI. Use of gentamicin for women with community-acquired uncomplicated acute pyelonephritis caused by gentamicin-susceptible or -resistant Escherichia coli: 10-year experience. Microb Drug Resist. 2013; 19:316–322.
Article9. Naber KG, Savov O, Salmen HC. Piperacillin 2 g/tazobactam 0.5 g is as effective as imipenem 0.5 g/cilastatin 0.5 g for the treatment of acute uncomplicated pyelonephritis and complicated urinary tract infections. Int J Antimicrob Agents. 2002; 19:95–103.
Article10. Safrin S, Siegel D, Black D. Pyelonephritis in adult women: inpatient versus outpatient therapy. Am J Med. 1988; 85:793–798.
Article11. Lim SK, Park IW, Lee WG, Kim HK, Choi YH. Change of antimicrobial susceptibility among Escherichia coli strains isolated from female patients with community-onset acute pyelonephritis. Yonsei Med J. 2012; 53:164–171.
Article12. Wie SH, Ki M, Kim J, Cho YK, Lim SK, Lee JS, et al. Clinical characteristics predicting early clinical failure after 72 h of antibiotic treatment in women with community-onset acute pyelonephritis: a prospective multicentre study. Clin Microbiol Infect. 2014; 20:O721–O729.
Article13. Ramakrishnan K, Scheid DC. Diagnosis and management of acute pyelonephritis in adults. Am Fam Physician. 2005; 71:933–942.14. Vazquez JA, González Patzán LD, Stricklin D, Duttaroy DD, Kreidly Z, Lipka J, et al. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin. 2012; 28:1921–1931.
Article15. Park DW, Peck KR, Chung MH, Lee JS, Park YS, Kim HY, et al. Comparison of ertapenem and ceftriaxone therapy for acute pyelonephritis and other complicated urinary tract infections in Korean adults: a randomized, double-blind, multicenter trial. J Korean Med Sci. 2012; 27:476–483.
Article16. Ronald AR, Harding GK. Complicated urinary tract infections. Infect Dis Clin North Am. 1997; 11:583–592.
Article17. York MK. Aerobic Bacteriology. In : Isenberg HD, editor. Clinical Microbiology Procedures Handbook. 2nd ed. Washington, DC: ASM press;2007. Section 3.12.1-3.12.15.18. Clinical and Laboratory Standard Institute. Performance standards for antimicrobial susceptibility testing: 15th informational supplement: approved standard MI00-S15. Wayne, PA: Clinical and Laboratory Standards Institute;2005.19. Shin J, Kim J, Wie SH, Cho YK, Lim SK, Shin SY, et al. Fluoroquinolone resistance in uncomplicated acute pyelonephritis: epidemiology and clinical impact. Microb Drug Resist. 2012; 18:169–175.
Article20. Pitout JD, Nordmann P, Laupland KB, Poirel L. Emergence of Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) in the community. J Antimicrob Chemother. 2005; 56:52–59.
Article21. Livermore DM, Hope R, Reynolds R, Blackburn R, Johnson AP, Woodford N. Declining cephalosporin and fluoroquinolone non-susceptibility among bloodstream Enterobacteriaceae from the UK: links to prescribing change? J Antimicrob Chemother. 2013; 68:2667–2674.
Article22. Spoorenberg V, Hulscher ME, Akkermans RP, Prins JM, Geerlings SE. Appropriate antibiotic use for patients with urinary tract infections reduces length of hospital stay. Clin Infect Dis. 2014; 58:164–169.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Change of Antimicrobial Susceptibility among Escherichia coli Strains Isolated from Female Patients with Community-Onset Acute Pyelonephritis
- National Trends of Antimicrobial Resistance in Uncomplicated Cystitis
- Use of cefuroxime for women with community-onset acute pyelonephritis caused by cefuroxime-susceptible or -resistant Escherichia coli
- A comparison of the clinical characteristics of elderly and non-elderly women with community-onset, non-obstructive acute pyelonephritis
- Treatment of Community-Acquired Uncomplicated Urinary Tract Infection