Yonsei Med J.
2015 Sep;56(5):1206-1212. 10.3349/ymj.2015.56.5.1206.
Prognostic Impacts of Metastatic Site and Pain on Progression to Castrate Resistance and Mortality in Patients with Metastatic Prostate Cancer
- Affiliations
-
- 1Department of Urology and Urological Science Institute, Yonsei University College of Medicine, Seoul, Korea. chung646@yuhs.ac
- KMID: 2163610
- DOI: http://doi.org/10.3349/ymj.2015.56.5.1206
Abstract
- PURPOSE
To investigate predictors of progression to castration-resistant prostate cancer (CRPC) and cancer-specific mortality (CSM) in patients with metastatic prostate cancer (mPCa).
MATERIALS AND METHODS
A retrospective analysis was performed on 440 consecutive treatment-naive patients initially diagnosed with mPCa between August 2000 and June 2012. Patient age, body mass index (BMI), Gleason score, prostate-specific antigen (PSA), PSA nadir, American Joint Committee on Cancer stage, Visual Analogue Scale pain score, Eastern Cooperative Oncology Group performance score (ECOG PS), PSA response to hormone therapy, and metastatic sites were assessed. Cox-proportional hazards regression analyses were used to evaluate survivals and predictive variables of men with bone metastasis stratified according to the presence of pain, compared to men with visceral metastasis.
RESULTS
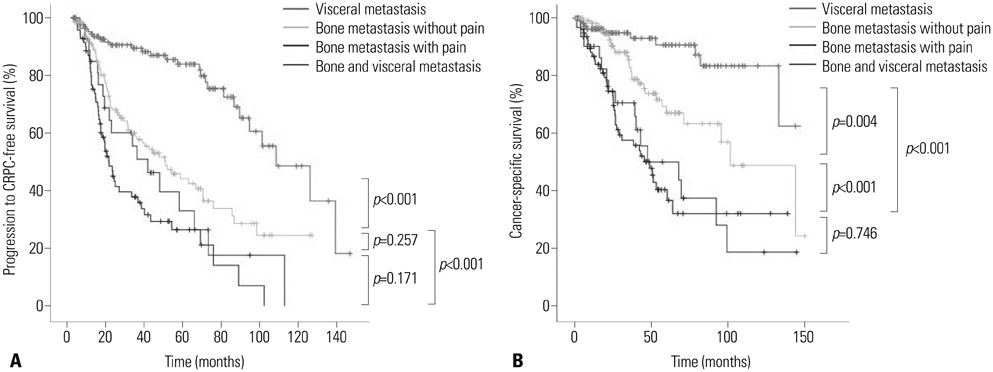
Metastases were most often found in bone (75.4%), followed by lung (16.3%) and liver (8.3%) tissues. Bone metastasis, pain, and high BMI were associated with increased risks of progression to CRPC, and bone metastasis, pain, PSA nadir, and ECOG PS> or =1 were significant predictors of CSM. During the median follow-up of 32.0 (interquartile range 14.7-55.9) months, patients with bone metastasis with pain and patients with both bone and visceral metastases showed the worst median progression to CRPC-free and cancer-specific survivals, followed by men with bone metastasis without pain. Patients with visceral metastasis had the best median survivals.
CONCLUSION
Metastatic spread and pain patterns confer different prognosis in patients with mPCa. Bone may serve as a crucial microenvironment in the development of CRPC and disease progression.
Keyword
MeSH Terms
-
Aged
Bone Neoplasms/secondary
*Disease Progression
Humans
Male
Middle Aged
Neoplasm Grading
Neoplasm Metastasis
Pain/diagnosis/etiology/prevention & control
Pain Measurement
Prognosis
Prostate-Specific Antigen/blood
Prostatic Neoplasms/mortality/*pathology
Prostatic Neoplasms, Castration-Resistant/mortality/*pathology
Retrospective Studies
Risk
Treatment Outcome
Prostate-Specific Antigen
Figure
Cited by 1 articles
-
Survival Outcomes of Concurrent Treatment with Docetaxel and Androgen Deprivation Therapy in Metastatic Castration-Resistant Prostate Cancer
Ho Seong Jang, Kyo Chul Koo, Kang Su Cho, Byung Ha Chung
Yonsei Med J. 2016;57(5):1070-1078. doi: 10.3349/ymj.2016.57.5.1070.
Reference
-
1. Mikel Hubanks J, Boorjian SA, Frank I, Gettman MT, Houston Thompson R, Rangel LJ, et al. The presence of extracapsular extension is associated with an increased risk of death from prostate cancer after radical prostatectomy for patients with seminal vesicle invasion and negative lymph nodes. Urol Oncol. 2014; 32:26.
Article2. Nørgaard M, Jensen AØ, Jacobsen JB, Cetin K, Fryzek JP, Sørensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol. 2010; 184:162–167.3. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006; 12(20 Pt 2):6243s–6249s.
Article4. Sturge J, Caley MP, Waxman J. Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat Rev Clin Oncol. 2011; 8:357–368.
Article5. Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004; 64:9209–9216.
Article6. Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989; 321:419–424.
Article7. Ryan CJ, Elkin EP, Cowan J, Carroll PR. Initial treatment patterns and outcome of contemporary prostate cancer patients with bone metastases at initial presentation: data from CaPSURE. Cancer. 2007; 110:81–86.
Article8. Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009; 101:878–887.
Article9. Chodak GW, Vogelzang NJ, Caplan RJ, Soloway M, Smith JA. Independent prognostic factors in patients with metastatic (stage D2) prostate cancer. The Zoladex Study Group. JAMA. 1991; 265:618–621.
Article10. Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003; 21:1232–1237.
Article11. Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008; 26:1148–1159.
Article12. Logothetis CJ, Navone NM, Lin SH. Understanding the biology of bone metastases: key to the effective treatment of prostate cancer. Clin Cancer Res. 2008; 14:1599–1602.
Article13. Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006; 106:1624–1633.
Article14. Loberg RD, Logothetis CJ, Keller ET, Pienta KJ. Pathogenesis and treatment of prostate cancer bone metastases: targeting the lethal phenotype. J Clin Oncol. 2005; 23:8232–8241.
Article15. Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol. 2011; 8:12–23.
Article16. Efstathiou E, Logothetis CJ. A new therapy paradigm for prostate cancer founded on clinical observations. Clin Cancer Res. 2010; 16:1100–1107.
Article17. laszczyk N, Masri BA, Mawji NR, Ueda T, McAlinden G, Duncan CP, et al. Osteoblast-derived factors induce androgen-independent proliferation and expression of prostate-specific antigen in human prostate cancer cells. Clin Cancer Res. 2004; 10:1860–1869.
Article18. Corn PG. The tumor microenvironment in prostate cancer: elucidating molecular pathways for therapy development. Cancer Manag Res. 2012; 4:183–193.
Article19. Achbarou A, Kaiser S, Tremblay G, Ste-Marie LG, Brodt P, Goltzman D, et al. Urokinase overproduction results in increased skeletal metastasis by prostate cancer cells in vivo. Cancer Res. 1994; 54:2372–2377.20. Koutsilieris M, Rabbani SA, Goltzman D. Effects of human prostatic mitogens on rat bone cells and fibroblasts. J Endocrinol. 1987; 115:447–454.
Article21. Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995; 1:944–949.
Article22. Lee GT, Kang DI, Ha YS, Jung YS, Chung J, Min K, et al. Prostate cancer bone metastases acquire resistance to androgen deprivation via WNT5A-mediated BMP-6 induction. Br J Cancer. 2014; 110:1634–1644.
Article23. Pezaro CJ, Omlin A, Lorente D, Nava Rodrigues D, Ferraldeschi R, Bianchini D, et al. Visceral disease in castration-resistant prostate cancer. Eur Urol. 2014; 65:270–273.24. Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013; 369:213–223.
Article25. Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999-2006. Prostate Cancer Prostatic Dis. 2011; 14:177–183.
Article26. Msaouel P, Nandikolla G, Pneumaticos SG, Koutsilieris M. Bone microenvironment-targeted manipulations for the treatment of osteoblastic metastasis in castration-resistant prostate cancer. Expert Opin Investig Drugs. 2013; 22:1385–1400.
Article27. Gandaglia G, Karakiewicz PI, Briganti A, Passoni NM, Schiffmann J, Trudeau V, et al. Impact of the Site of Metastases on Survival in Patients with Metastatic Prostate Cancer. Eur Urol. 2014; Aug. 6. [Epub]. DOI: 10.1016/j.eururo.2014.07.020.
Article28. Hong SY, Cho DS, Kim SI, Ahn HS, Kim SJ. Prostate-specific antigen nadir and time to prostate-specific antigen nadir following maximal androgen blockade independently predict prognosis in patients with metastatic prostate cancer. Korean J Urol. 2012; 53:607–613.
Article29. Morote J, Esquena S, Abascal JM, Trilla E, Cecchini L, Raventós CX, et al. Usefulness of prostate-specific antigen nadir as predictor of androgen-independent progression of metastatic prostate cancer. Int J Biol Markers. 2005; 20:209–216.
Article30. Park JM, Nam JS, Na W, Oh JJ, Lee S, Hong SK, et al. Prognostic value of body mass index in Korean patients with castration-resistant prostate cancer. Korean J Urol. 2012; 53:761–765.
Article31. Bub JD, Miyazaki T, Iwamoto Y. Adiponectin as a growth inhibitor in prostate cancer cells. Biochem Biophys Res Commun. 2006; 340:1158–1166.
Article32. Briganti A, Suardi N, Gallina A, Abdollah F, Novara G, Ficarra V, et al. Predicting the risk of bone metastasis in prostate cancer. Cancer Treat Rev. 2014; 40:3–11.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 68Ga-PSMA Uptake in Subchondral Cyst Giving a False Impression of Disease Progression after 177Lu-PSMA Radioligand Therapy in Metastatic Castrate-Resistant Prostate Cancer
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Animal models of bone metastatic prostate cancer
- The Factors Affecting Prognosis in Patients with Metastatic Prostate Cancer after Hormonal Therapy
- Prognostic Significance of Prostate-specific Antigen Level Two Months after Maximal Androgen Blockade in Metastatic Prostate Cancer