Diabetes Metab J.
2016 Apr;40(2):99-114. 10.4093/dmj.2016.40.2.99.
An Update on the Effect of Incretin-Based Therapies on β-Cell Function and Mass
- Affiliations
-
- 1Department of Endocrinology and Metabolism, Kyung Hee University School of Medicine, Seoul, Korea.
- 2Department of Diabetes and Endocrinology, DHU FIRE, Lariboisière Hospital, University Paris-Diderot Paris-7, Paris, France. jean-francois.gautier@lrb.aphp.fr
- 3Clinical Investigation Center, INSERM-CIC9504, Saint-Louis University Hospital, University Paris-Diderot Paris-7, Paris, France.
- 4INSERM UMRS 1138, Cordeliers Research Center, University Pierre et Marie Curie Paris-6, Paris, France.
- KMID: 2162081
- DOI: http://doi.org/10.4093/dmj.2016.40.2.99
Abstract
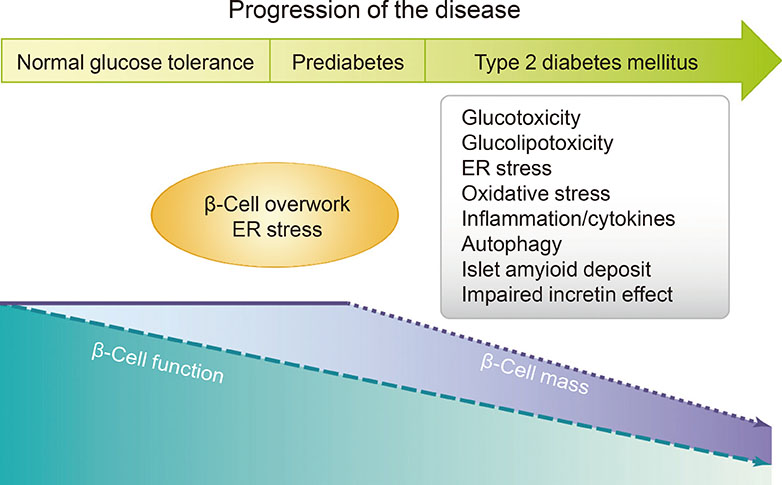
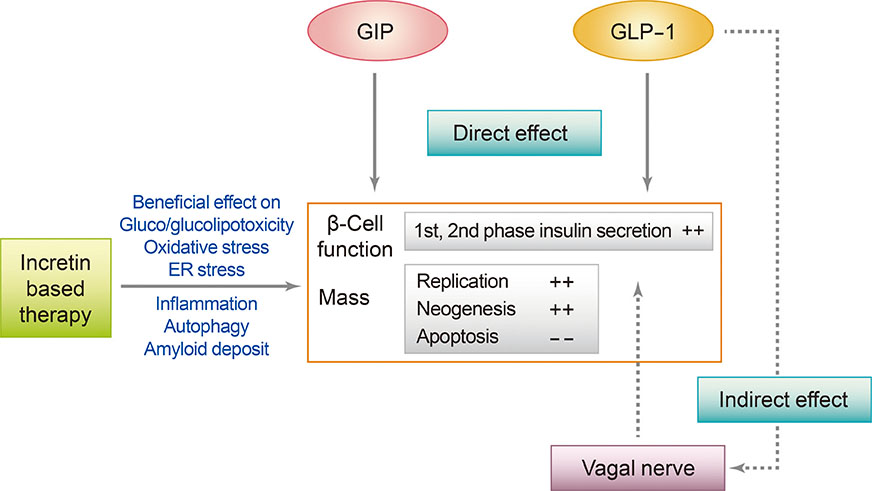
- Type 2 diabetes mellitus (T2DM) is a multifactorial disease with a complex and progressive pathogenesis. The two primary mechanisms of T2DM pathogenesis are pancreatic β-cell dysfunction and insulin resistance. Pancreatic β-cell dysfunction is recognized to be a prerequisite for the development of T2DM. Therapeutic modalities that improve β-cell function are considered critical to T2DM management; however, blood glucose control remains a challenge for many patients due to suboptimal treatment efficacy and the progressive nature of T2DM. Incretin-based therapies are now the most frequently prescribed antidiabetic drugs in Korea. Incretin-based therapies are a favorable class of drugs due to their ability to reduce blood glucose by targeting the incretin hormone system and, most notably, their potential to improve pancreatic β-cell function. This review outlines the current understanding of the incretin hormone system in T2DM and summarizes recent updates on the effect of incretin-based therapies on β-cell function and β-cell mass in animals and humans.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Assessment of Insulin Secretion and Insulin Resistance in Human
So Young Park, Jean-François Gautier, Suk Chon
Diabetes Metab J. 2021;45(5):641-654. doi: 10.4093/dmj.2021.0220.Incretin and Pancreatic β-Cell Function in Patients with Type 2 Diabetes
Chang Ho Ahn, Tae Jung Oh, Se Hee Min, Young Min Cho
Endocrinol Metab. 2023;38(1):1-9. doi: 10.3803/EnM.2023.103.
Reference
-
1. Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009; 58:773–795.2. U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995; 44:1249–1258.3. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006; 355:2427–2443.4. Chon S, Riveline JP, Blondeau B, Gautier JF. Incretin-based therapy and pancreatic beta cells. Diabetes Metab. 2014; 40:411–422.5. Zunz E, La Barre J. Contributiona a l'etude des variations physiologiques de la secretion interne du pancreas: relations entre les secretions externe et interne du pancreas. Arch Int Physiol Biochim. 1929; 31:20–44.6. Elrick H, Stimmler L, Hlad CJ Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964; 24:1076–1082.7. Yabe D, Seino Y. Two incretin hormones GLP-1 and GIP: comparison of their actions in insulin secretion and β cell preservation. Prog Biophys Mol Biol. 2011; 107:248–256.8. Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003; 46:1029–1045.9. Luzi L, DeFronzo RA. Effect of loss of first-phase insulin secretion on hepatic glucose production and tissue glucose disposal in humans. Am J Physiol. 1989; 257(2 Pt 1):E241–E246.10. Steiner KE, Mouton SM, Bowles CR, Williams PE, Cherrington AD. The relative importance of first- and second-phase insulin secretion in countering the action of glucagon on glucose turnover in the conscious dog. Diabetes. 1982; 31:964–972.11. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006; 3:153–165.12. Meloni AR, DeYoung MB, Lowe C, Parkes DG. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes Metab. 2013; 15:15–27.13. Fehmann HC, Goke R, Goke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr Rev. 1995; 16:390–410.14. Holz GG. Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes. 2004; 53:5–13.15. Phillips LK, Prins JB. Update on incretin hormones. Ann N Y Acad Sci. 2011; 1243:E55–E74.16. Burcelin R, Da Costa A, Drucker D, Thorens B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes. 2001; 50:1720–1728.17. Cabou C, Burcelin R. GLP-1, the gut-brain, and brain-periphery axes. Rev Diabet Stud. 2011; 8:418–431.18. Ahren B. Sensory nerves contribute to insulin secretion by glucagon-like peptide-1 in mice. Am J Physiol Regul Integr Comp Physiol. 2004; 286:R269–R272.19. Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, Benhamed F, Gremeaux T, Drucker DJ, Kahn CR, Girard J, Tanti JF, Delzenne NM, Postic C, Burcelin R. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest. 2005; 115:3554–3563.20. Dalle S, Burcelin R, Gourdy P. Specific actions of GLP-1 receptor agonists and DPP4 inhibitors for the treatment of pancreatic β-cell impairments in type 2 diabetes. Cell Signal. 2013; 25:570–579.21. Widenmaier SB, Ao Z, Kim SJ, Warnock G, McIntosh CH. Suppression of p38 MAPK and JNK via Akt-mediated inhibition of apoptosis signal-regulating kinase 1 constitutes a core component of the beta-cell pro-survival effects of glucose-dependent insulinotropic polypeptide. J Biol Chem. 2009; 284:30372–30382.22. Klinger S, Poussin C, Debril MB, Dolci W, Halban PA, Thorens B. Increasing GLP-1-induced beta-cell proliferation by silencing the negative regulators of signaling cAMP response element modulator-alpha and DUSP14. Diabetes. 2008; 57:584–593.23. Porte D Jr. Clinical importance of insulin secretion and its interaction with insulin resistance in the treatment of type 2 diabetes mellitus and its complications. Diabetes Metab Res Rev. 2001; 17:181–188.24. Ferner RE, Ashworth L, Tronier B, Alberti KG. Effects of short-term hyperglycemia on insulin secretion in normal humans. Am J Physiol. 1986; 250(6 Pt 1):E655–E661.25. Guillausseau PJ, Meas T, Virally M, Laloi-Michelin M, Medeau V, Kevorkian JP. Abnormalities in insulin secretion in type 2 diabetes mellitus. Diabetes Metab. 2008; 34:Suppl 2. S43–S48.26. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999; 104:787–794.27. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003; 52:102–110.28. Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003; 88:2300–2308.29. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008; 10:Suppl 4. 32–42.30. Meier JJ, Breuer TG, Bonadonna RC, Tannapfel A, Uhl W, Schmidt WE, Schrader H, Menge BA. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia. 2012; 55:1346–1354.31. Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest. 1984; 74:1318–1328.32. Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003; 52:581–587.33. Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995; 44:863–870.34. Prentki M, Joly E, El-Assaad W, Roduit R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in beta-cell adaptation and failure in the etiology of diabetes. Diabetes. 2002; 51:Suppl 3. S405–S413.35. Ahren B. Incretin dysfunction in type 2 diabetes: clinical impact and future perspectives. Diabetes Metab. 2013; 39:195–201.36. Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med. 2011; 124:1 Suppl. S3–S18.37. Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia. 2011; 54:10–18.38. Hansen KB, Vilsboll T, Bagger JI, Holst JJ, Knop FK. Impaired incretin-induced amplification of insulin secretion after glucose homeostatic dysregulation in healthy subjects. J Clin Endocrinol Metab. 2012; 97:1363–1370.39. Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015; 4:212283.40. Barnett AH. Lixisenatide: evidence for its potential use in the treatment of type 2 diabetes. Core Evid. 2011; 6:67–79.41. Wang C, Chen X, Ding X, He Y, Gu C, Zhou L. Exendin-4 promotes beta cell proliferation via PI3k/Akt signalling pathway. Cell Physiol Biochem. 2015; 35:2223–2232.42. Zhao Q, Yang Y, Hu J, Shan Z, Wu Y, Lei L. Exendin-4 enhances expression of Neurod1 and Glut2 in insulin-producing cells derived from mouse embryonic stem cells. Arch Med Sci. 2016; 12:199–207.43. Abe H, Uchida T, Hara A, Mizukami H, Komiya K, Koike M, Shigihara N, Toyofuku Y, Ogihara T, Uchiyama Y, Yagihashi S, Fujitani Y, Watada H. Exendin-4 improves β-cell function in autophagy-deficient β-cells. Endocrinology. 2013; 154:4512–4524.44. Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, Knudsen LB. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002; 283:E745–E752.45. Bregenholt S, Moldrup A, Blume N, Karlsen AE, Nissen Friedrichsen B, Tornhave D, Knudsen LB, Petersen JS. The long-acting glucagon-like peptide-1 analogue, liraglutide, inhibits beta-cell apoptosis in vitro. Biochem Biophys Res Commun. 2005; 330:577–584.46. Shimoda M, Kanda Y, Hamamoto S, Tawaramoto K, Hashiramoto M, Matsuki M, Kaku K. The human glucagon-like peptide-1 analogue liraglutide preserves pancreatic beta cells via regulation of cell kinetics and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetologia. 2011; 54:1098–1108.47. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013; 17:819–837.48. Ellenbroek JH, Tons HA, Westerouen van Meeteren MJ, de Graaf N, Hanegraaf MA, Rabelink TJ, Carlotti F, de Koning EJ. Glucagon-like peptide-1 receptor agonist treatment reduces beta cell mass in normoglycaemic mice. Diabetologia. 2013; 56:1980–1986.49. Zheng J, Chen T, Zhu Y, Li HQ, Deng XL, Wang QH, Zhang JY, Chen LL. Liraglutide prevents fast weight gain and β-cell dysfunction in male catch-up growth rats. Exp Biol Med (Maywood). 2015; 240:1165–1176.50. Koehler JA, Baggio LL, Cao X, Abdulla T, Campbell JE, Secher T, Jelsing J, Larsen B, Drucker DJ. Glucagon-like peptide-1 receptor agonists increase pancreatic mass by induction of protein synthesis. Diabetes. 2015; 64:1046–1056.51. Yang C, Loehn M, Jurczyk A, Przewozniak N, Leehy L, Herrera PL, Shultz LD, Greiner DL, Harlan DM, Bortell R. Lixisenatide accelerates restoration of normoglycemia and improves human beta-cell function and survival in diabetic immunodeficient NOD-scid IL-2rg(null) RIP-DTR mice engrafted with human islets. Diabetes Metab Syndr Obes. 2015; 8:387–398.52. Tian L, Gao J, Weng G, Yi H, Tian B, O'Brien TD, Guo Z. Comparison of exendin-4 on beta-cell replication in mouse and human islet grafts. Transpl Int. 2011; 24:856–864.53. He ZX, Zhou ZW, Yang Y, Yang T, Pan SY, Qiu JX, Zhou SF. Overview of clinically approved oral antidiabetic agents for the treatment of type 2 diabetes mellitus. Clin Exp Pharmacol Physiol. 2015; 42:125–138.54. van Genugten RE, van Raalte DH, Diamant M. Dipeptidyl peptidase-4 inhibitors and preservation of pancreatic islet-cell function: a critical appraisal of the evidence. Diabetes Obes Metab. 2012; 14:101–111.55. Flock G, Baggio LL, Longuet C, Drucker DJ. Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes. 2007; 56:3006–3013.56. Hamamoto S, Kanda Y, Shimoda M, Tatsumi F, Kohara K, Tawaramoto K, Hashiramoto M, Kaku K. Vildagliptin preserves the mass and function of pancreatic β cells via the developmental regulation and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetes Obes Metab. 2013; 15:153–163.57. Omar BA, Vikman J, Winzell MS, Voss U, Ekblad E, Foley JE, Ahren B. Enhanced beta cell function and anti-inflammatory effect after chronic treatment with the dipeptidyl peptidase-4 inhibitor vildagliptin in an advanced-aged diet-induced obesity mouse model. Diabetologia. 2013; 56:1752–1760.58. Wu YJ, Guo X, Li CJ, Li DQ, Zhang J, Yang Y, Kong Y, Guo H, Liu DM, Chen LM. Dipeptidyl peptidase-4 inhibitor, vildagliptin, inhibits pancreatic beta cell apoptosis in association with its effects suppressing endoplasmic reticulum stress in db/db mice. Metabolism. 2015; 64:226–235.59. Shah P, Ardestani A, Dharmadhikari G, Laue S, Schumann DM, Kerr-Conte J, Pattou F, Klein T, Maedler K. The DPP-4 inhibitor linagliptin restores β-cell function and survival in human isolated islets through GLP-1 stabilization. J Clin Endocrinol Metab. 2013; 98:E1163–E1172.60. Matveyenko AV, Dry S, Cox HI, Moshtaghian A, Gurlo T, Galasso R, Butler AE, Butler PC. Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes. 2009; 58:1604–1615.61. Morita A, Mukai E, Hiratsuka A, Takatani T, Iwanaga T, Lee EY, Miki T. Distinct effects of dipeptidyl peptidase-4 inhibitor and glucagon-like peptide-1 receptor agonist on islet morphology and function. Endocrine. 2016; 51:429–439.62. Campbell RK. Clarifying the role of incretin-based therapies in the treatment of type 2 diabetes mellitus. Clin Ther. 2011; 33:511–527.63. Bunck MC, Diamant M, Corner A, Eliasson B, Malloy JL, Shaginian RM, Deng W, Kendall DM, Taskinen MR, Smith U, Yki-Jarvinen H, Heine RJ. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009; 32:762–768.64. Chang AM, Jakobsen G, Sturis J, Smith MJ, Bloem CJ, An B, Galecki A, Halter JB. The GLP-1 derivative NN2211 restores beta-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes. 2003; 52:1786–1791.65. Vilsboll T, Brock B, Perrild H, Levin K, Lervang HH, Kolendorf K, Krarup T, Schmitz O, Zdravkovic M, Le-Thi T, Madsbad S. Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with type 2 diabetes mellitus. Diabet Med. 2008; 25:152–156.66. Madsbad S. Exenatide and liraglutide: different approaches to develop GLP-1 receptor agonists (incretin mimetics). Preclinical and clinical results. Best Pract Res Clin Endocrinol Metab. 2009; 23:463–477.67. Bolli GB, Munteanu M, Dotsenko S, Niemoeller E, Boka G, Wu Y, Hanefeld M. Efficacy and safety of lixisenatide once daily vs. placebo in people with type 2 diabetes insufficiently controlled on metformin (GetGoal-F1). Diabet Med. 2014; 31:176–184.68. Kapitza C, Forst T, Coester HV, Poitiers F, Ruus P, Hincelin Mery A. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab. 2013; 15:642–649.69. Edwards KL, Minze MG. Dulaglutide: an evidence-based review of its potential in the treatment of type 2 diabetes. Core Evid. 2015; 10:11–21.70. Bunck MC, Corner A, Eliasson B, Heine RJ, Shaginian RM, Taskinen MR, Smith U, Yki-Jarvinen H, Diamant M. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care. 2011; 34:2041–2047.71. Gallwitz B, Guzman J, Dotta F, Guerci B, Simo R, Basson BR, Festa A, Kiljanski J, Sapin H, Trautmann M, Schernthaner G. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet. 2012; 379:2270–2278.72. Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B. LEAD-3 (Mono) Study Group. Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab. 2011; 13:348–356.73. Mari A, Scherbaum WA, Nilsson PM, Lalanne G, Schweizer A, Dunning BE, Jauffret S, Foley JE. Characterization of the influence of vildagliptin on model-assessed -cell function in patients with type 2 diabetes and mild hyperglycemia. J Clin Endocrinol Metab. 2008; 93:103–109.74. Aaboe K, Knop FK, Vilsboll T, Deacon CF, Holst JJ, Madsbad S, Krarup T. Twelve weeks treatment with the DPP-4 inhibitor, sitagliptin, prevents degradation of peptide YY and improves glucose and non-glucose induced insulin secretion in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2010; 12:323–333.75. Scherbaum WA, Schweizer A, Mari A, Nilsson PM, Lalanne G, Jauffret S, Foley JE. Efficacy and tolerability of vildagliptin in drug-naive patients with type 2 diabetes and mild hyperglycaemia. Diabetes Obes Metab. 2008; 10:675–682.76. Ahren B, Pacini G, Foley JE, Schweizer A. Improved meal-related beta-cell function and insulin sensitivity by the dipeptidyl peptidase-IV inhibitor vildagliptin in metformin-treated patients with type 2 diabetes over 1 year. Diabetes Care. 2005; 28:1936–1940.77. Foley JE, Bunck MC, Moller-Goede DL, Poelma M, Nijpels G, Eekhoff EM, Schweizer A, Heine RJ, Diamant M. Beta cell function following 1 year vildagliptin or placebo treatment and after 12 week washout in drug-naïve patients with type 2 diabetes and mild hyperglycaemia: a randomised controlled trial. Diabetologia. 2011; 54:1985–1991.78. Esposito K, Chiodini P, Maiorino MI, Bellastella G, Capuano A, Giugliano D. Glycaemic durability with dipeptidyl peptidase-4 inhibitors in type 2 diabetes: a systematic review and meta-analysis of long-term randomised controlled trials. BMJ Open. 2014; 4:e005442.79. Leibowitz G, Cahn A, Bhatt DL, Hirshberg B, Mosenzon O, Wei C, Jermendy G, Sheu WH, Sendon JL, Im K, Braunwald E, Scirica BM, Raz I. Impact of treatment with saxagliptin on glycaemic stability and β-cell function in the SAVOR-TIMI 53 study. Diabetes Obes Metab. 2015; 17:487–494.80. Buzzetti R, Pozzilli P, Frederich R, Iqbal N, Hirshberg B. Saxagliptin improves glycaemic control and C-peptide secretion in latent autoimmune diabetes in adults (LADA). Diabetes Metab Res Rev. 2016; 32:289–296.81. Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Response to comments on: Butler et al. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013; 62:2595–2604.82. Kahn SE. Incretin therapy and islet pathology: a time for caution. Diabetes. 2013; 62:2178–2180.83. Harja E, Lord J, Skyler JS. An analysis of characteristics of subjects examined for incretin effects on pancreatic pathology. Diabetes Technol Ther. 2013; 15:609–618.84. Bonner-Weir S, In't Veld PA, Weir GC. Reanalysis of study of pancreatic effects of incretin therapy: methodological deficiencies. Diabetes Obes Metab. 2014; 16:661–666.85. Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, Rosebraugh C. Pancreatic safety of incretin-based drugs: FDA and EMA assessment. N Engl J Med. 2014; 370:794–797.86. Sheffield CA, Kane MP, Busch RS. Off-label use of exenatide for the management of insulin-resistant type 1 diabetes mellitus in an obese patient with human immunodeficiency virus infection. Pharmacotherapy. 2007; 27:1449–1455.87. Dupre J, Behme MT, McDonald TJ. Exendin-4 normalized postcibal glycemic excursions in type 1 diabetes. J Clin Endocrinol Metab. 2004; 89:3469–3473.88. Rother KI, Spain LM, Wesley RA, Digon BJ 3rd, Baron A, Chen K, Nelson P, Dosch HM, Palmer JP, Brooks-Worrell B, Ring M, Harlan DM. Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care. 2009; 32:2251–2257.89. Dejgaard TF, Frandsen CS, Hansen TS, Almdal T, Urhammer S, Pedersen-Bjergaard U, Jensen T, Jensen AK, Holst JJ, Tarnow L, Knop FK, Madsbad S, Andersen HU. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2016; 4:221–232.90. Hari Kumar KV, Shaikh A, Prusty P. Addition of exenatide or sitagliptin to insulin in new onset type 1 diabetes: a randomized, open label study. Diabetes Res Clin Pract. 2013; 100:e55–e58.91. Ghofaili KA, Fung M, Ao Z, Meloche M, Shapiro RJ, Warnock GL, Elahi D, Meneilly GS, Thompson DM. Effect of exenatide on beta cell function after islet transplantation in type 1 diabetes. Transplantation. 2007; 83:24–28.92. Faradji RN, Froud T, Messinger S, Monroy K, Pileggi A, Mineo D, Tharavanij T, Mendez AJ, Ricordi C, Alejandro R. Long-term metabolic and hormonal effects of exenatide on islet transplant recipients with allograft dysfunction. Cell Transplant. 2009; 18:1247–1259.93. Gangemi A, Salehi P, Hatipoglu B, Martellotto J, Barbaro B, Kuechle JB, Qi M, Wang Y, Pallan P, Owens C, Bui J, West D, Kaplan B, Benedetti E, Oberholzer J. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. 2008; 8:1250–1261.94. Cechin SR, Perez-Alvarez I, Fenjves E, Molano RD, Pileggi A, Berggren PO, Ricordi C, Pastori RL. Anti-inflammatory properties of exenatide in human pancreatic islets. Cell Transplant. 2012; 21:633–648.95. Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, Madeira MI, Malmegrim KC, Foss-Freitas MC, Simoes BP, Martinez EZ, Foss MC, Burt RK, Voltarelli JC. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009; 301:1573–1579.96. Kim SJ, Nian C, Doudet DJ, McIntosh CH. Dipeptidyl peptidase IV inhibition with MK0431 improves islet graft survival in diabetic NOD mice partially via T-cell modulation. Diabetes. 2009; 58:641–651.97. Levine F, Itkin-Ansari P. Beta-cell regeneration: neogenesis, replication or both? J Mol Med (Berl). 2008; 86:247–258.98. Parnaud G, Bosco D, Berney T, Pattou F, Kerr-Conte J, Donath MY, Bruun C, Mandrup-Poulsen T, Billestrup N, Halban PA. Proliferation of sorted human and rat beta cells. Diabetologia. 2008; 51:91–100.99. Tiedge M. Inside the pancreas: progress and challenges of human beta cell mass quantification. Diabetologia. 2014; 57:856–859.100. Nakashima R, Yano T, Ogawa J, Tanaka N, Toda N, Yoshida M, Takano R, Inoue M, Honda T, Kume S, Matsumoto K. Potentiation of insulin secretion and improvement of glucose intolerance by combining a novel G protein-coupled receptor 40 agonist DS-1558 with glucagon-like peptide-1 receptor agonists. Eur J Pharmacol. 2014; 737:194–201.101. Dalboge LS, Almholt DL, Neerup TS, Vrang N, Jelsing J, Fosgerau K. The novel GLP-1-gastrin dual agonist ZP3022 improves glucose homeostasis and increases β-cell mass without affecting islet number in db/db mice. J Pharmacol Exp Ther. 2014; 350:353–360.102. Ansarullah , Lu Y, Holstein M, DeRuyter B, Rabinovitch A, Guo Z. Stimulating β-cell regeneration by combining a GPR119 agonist with a DPP-IV inhibitor. PLoS One. 2013; 8:e53345.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Injectable Drugs in Diabetes Treatment: Insulin versus Incretin
- Incretin-Based Therapies: A Promising Approach for Modulating Oxidative Stress and Insulin Resistance in Sarcopenia
- In Vivo Models for Incretin Research: From the Intestine to the Whole Body
- New Therapeutics for Diabetes Using Incretin Hormone
- Incretin-based Combination Therapy in Type 2 Diabetes Mellitus