Korean J Radiol.
2015 Oct;16(5):973-985. 10.3348/kjr.2015.16.5.973.
Whole-Body MRI in Children: Current Imaging Techniques and Clinical Applications
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea. hwgoo@amc.seoul.kr
- KMID: 2160764
- DOI: http://doi.org/10.3348/kjr.2015.16.5.973
Abstract
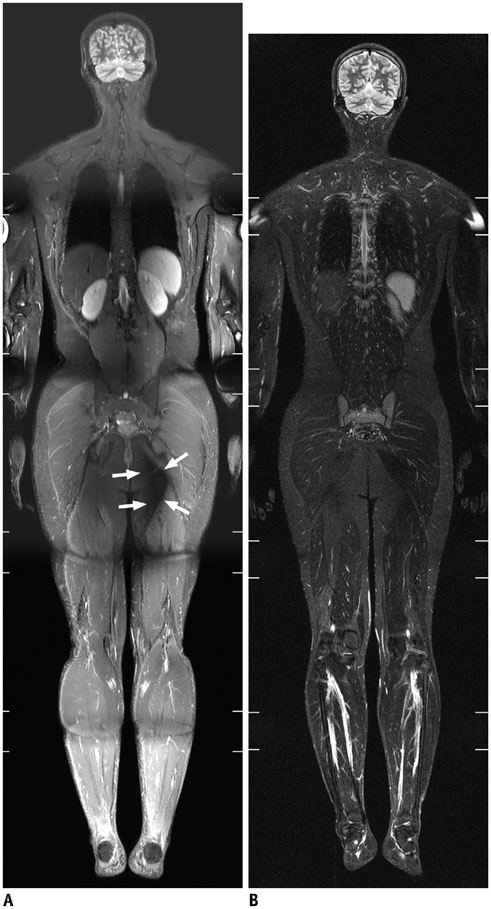
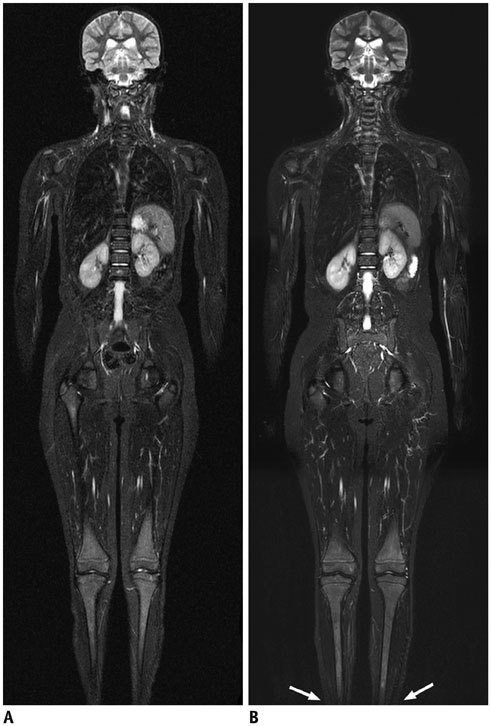
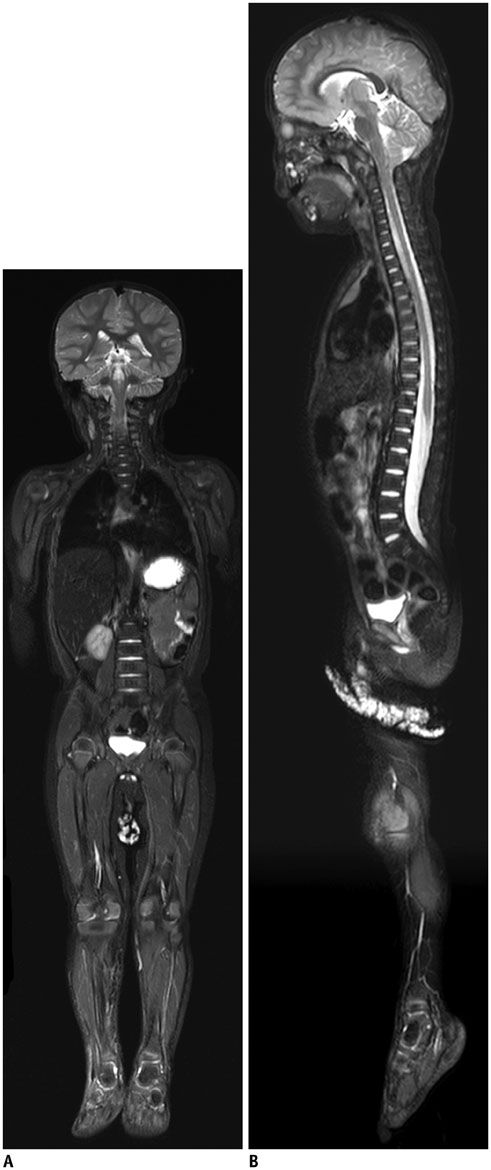
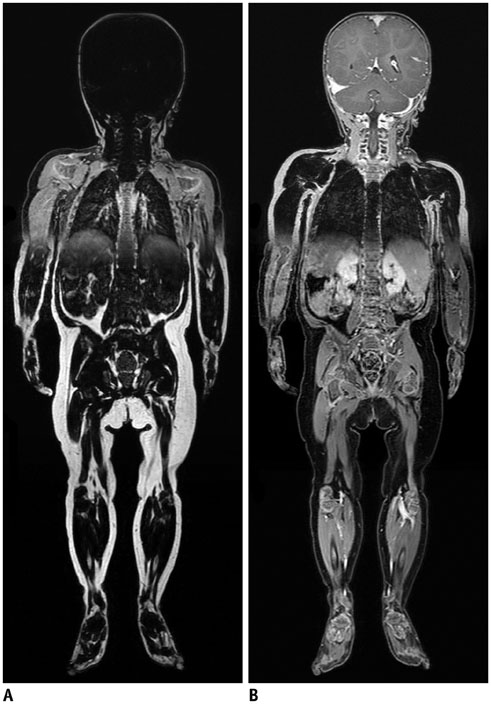
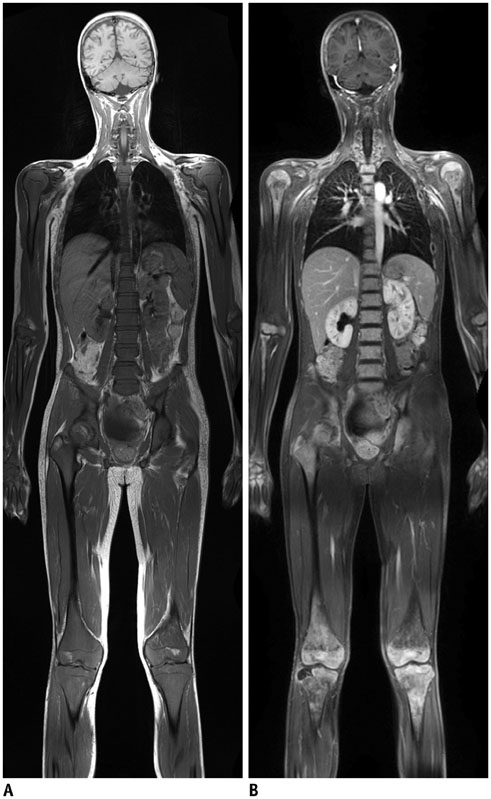
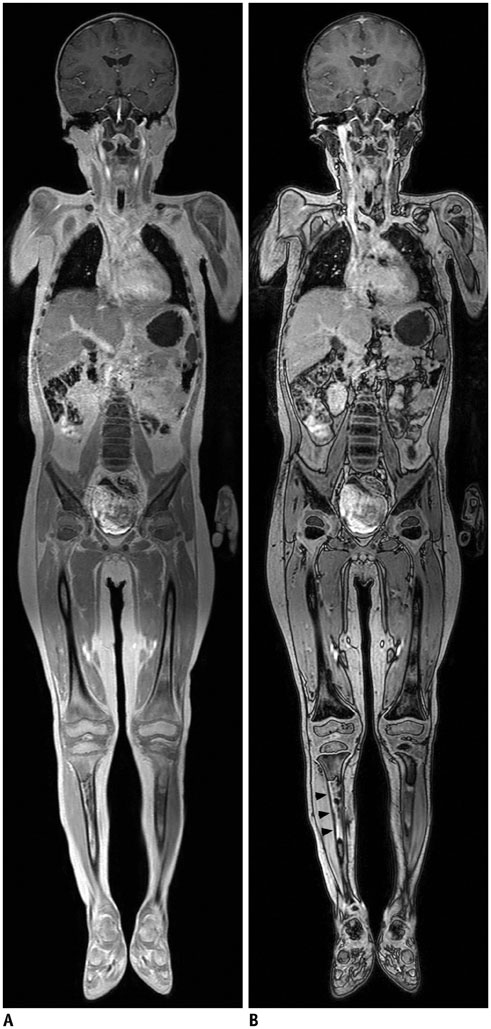
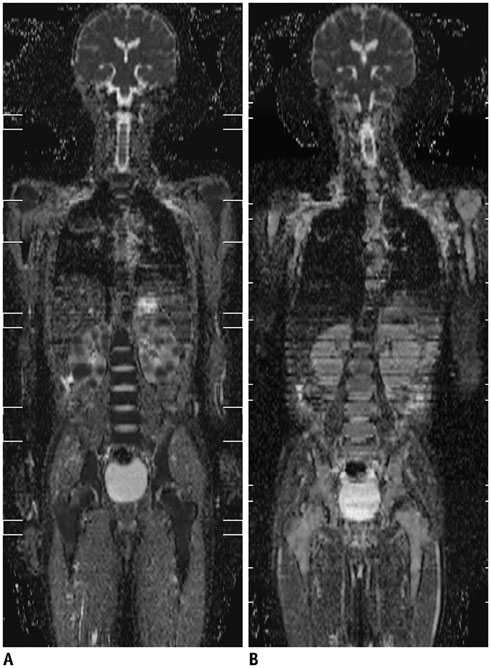
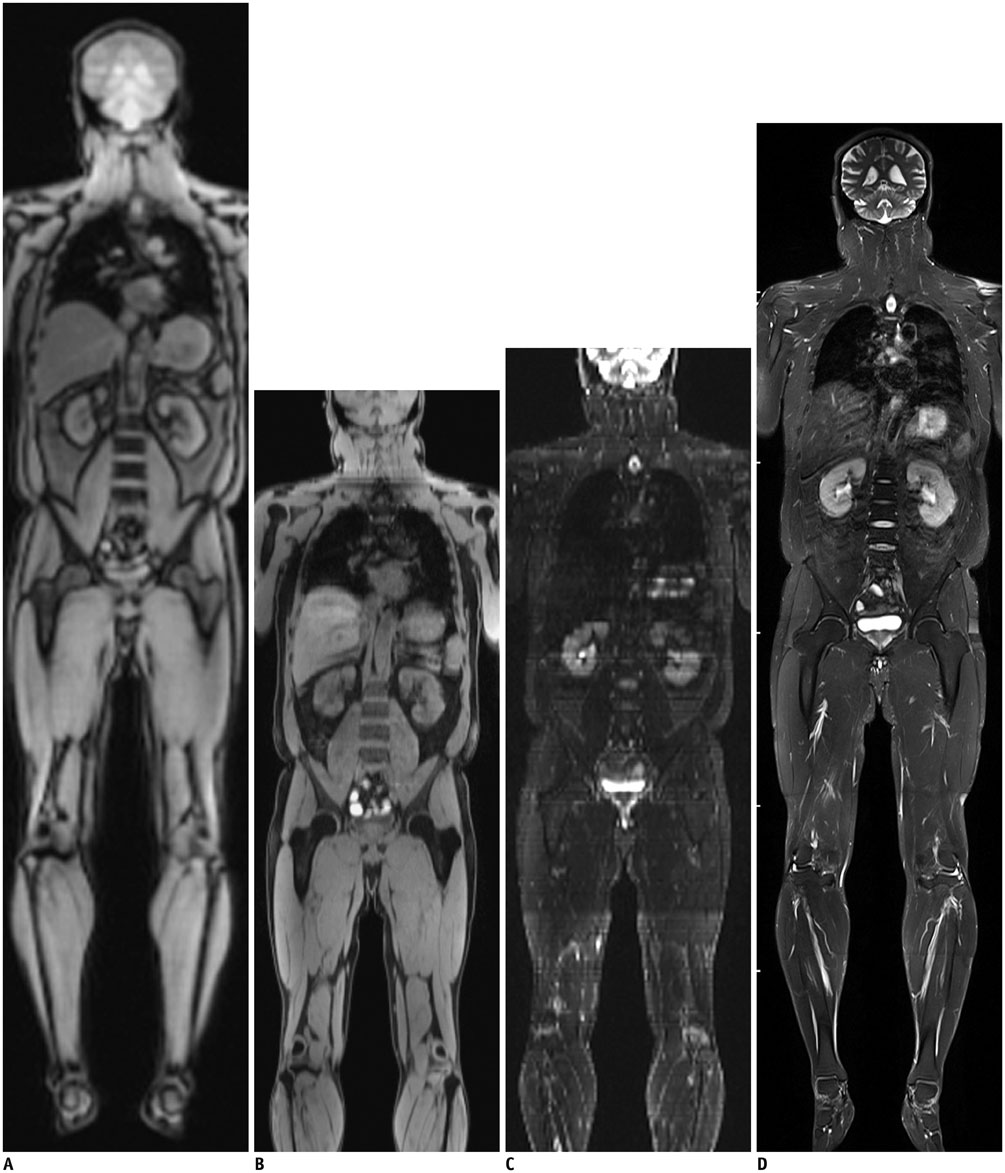
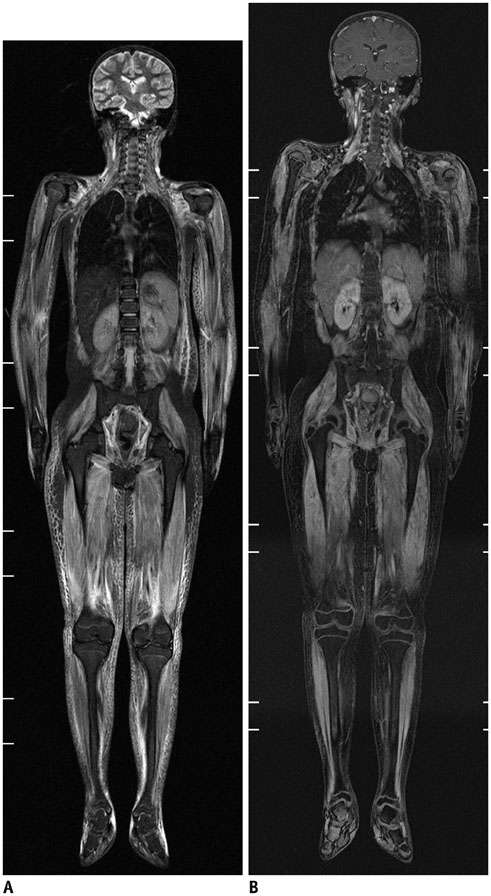
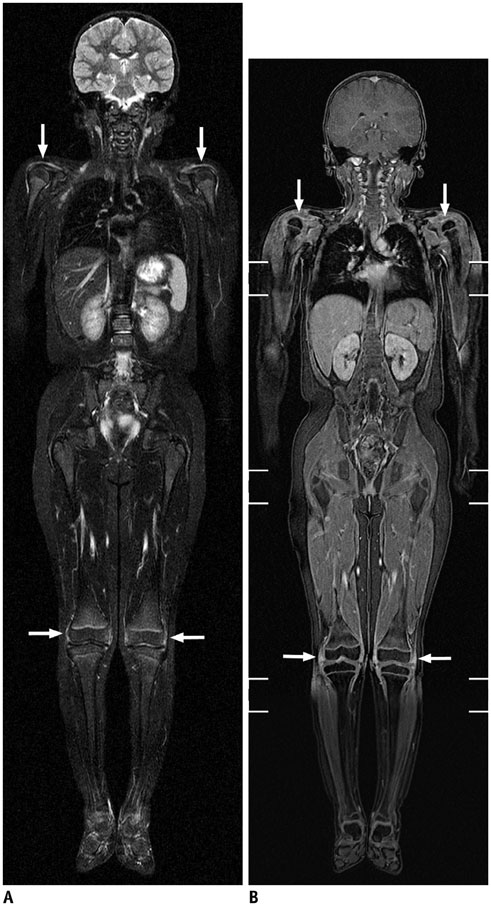
- Whole-body magnetic resonance imaging (MRI) is increasingly used in children to evaluate the extent and distribution of various neoplastic and non-neoplastic diseases. Not using ionizing radiation is a major advantage of pediatric whole-body MRI. Coronal and sagittal short tau inversion recovery imaging is most commonly used as the fundamental whole-body MRI protocol. Diffusion-weighted imaging and Dixon-based imaging, which has been recently incorporated into whole-body MRI, are promising pulse sequences, particularly for pediatric oncology. Other pulse sequences may be added to increase diagnostic capability of whole-body MRI. Of importance, the overall whole-body MRI examination time should be less than 30-60 minutes in children, regardless of the imaging protocol. Established and potentially useful clinical applications of pediatric whole-body MRI are described.
Keyword
MeSH Terms
Figure
Reference
-
1. Daldrup-Link HE, Franzius C, Link TM, Laukamp D, Sciuk J, Jürgens H, et al. Whole-body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG PET. AJR Am J Roentgenol. 2001; 177:229–236.2. Goo HW, Choi SH, Ghim T, Moon HN, Seo JJ. Whole-body MRI of paediatric malignant tumours: comparison with conventional oncological imaging methods. Pediatr Radiol. 2005; 35:766–773.3. Goo HW, Yang DH, Ra YS, Song JS, Im HJ, Seo JJ, et al. Whole-body MRI of Langerhans cell histiocytosis: comparison with radiography and bone scintigraphy. Pediatr Radiol. 2006; 36:1019–1031.4. Punwani S, Taylor SA, Bainbridge A, Prakash V, Bandula S, De Vita E, et al. Pediatric and adolescent lymphoma: comparison of whole-body STIR half-Fourier RARE MR imaging with an enhanced PET/CT reference for initial staging. Radiology. 2010; 255:182–190.5. Goo HW. Whole-body MRI of neuroblastoma. Eur J Radiol. 2010; 75:306–314.6. Chavhan GB, Babyn PS. Whole-body MR imaging in children: principles, technique, current applications, and future directions. Radiographics. 2011; 31:1757–1772.7. Goo HW. Regional and whole-body imaging in pediatric oncology. Pediatr Radiol. 2011; 41:Suppl 1. S186–S194.8. Goo HW. High field strength magnetic resonance imaging in children. J Korean Med Assoc. 2010; 53:1093–1102.9. Willinek WA, Gieseke J, Kukuk GM, Nelles M, König R, Morakkabati-Spitz N, et al. Dual-source parallel radiofrequency excitation body MR imaging compared with standard MR imaging at 3.0 T: initial clinical experience. Radiology. 2010; 256:966–975.10. Takahara T, Kwee T, Kibune S, Ochiai R, Sakamoto T, Niwa T, et al. Whole-body MRI using a sliding table and repositioning surface coil approach. Eur Radiol. 2010; 20:1366–1373.11. Brandão S, Seixas D, Ayres-Basto M, Castro S, Neto J, Martins C, et al. Comparing T1-weighted and T2-weighted three-point Dixon technique with conventional T1-weighted fat-saturation and short-tau inversion recovery (STIR) techniques for the study of the lumbar spine in a short-bore MRI machine. Clin Radiol. 2013; 68:e617–e623.12. Costelloe CM, Madewell JE, Kundra V, Harrell RK, Bassett RL Jr, Ma J. Conspicuity of bone metastases on fast Dixon-based multisequence whole-body MRI: clinical utility per sequence. Magn Reson Imaging. 2013; 31:669–675.13. Schmidt MA. Phase-uncertainty quality map for two-point Dixon fat-water separation. Phys Med Biol. 2011; 56:N195–N205.14. Pasoglou V, Michoux N, Peeters F, Larbi A, Tombal B, Selleslagh T, et al. Whole-body 3D T1-weighted MR imaging in patients with prostate cancer: feasibility and evaluation in screening for metastatic disease. Radiology. 2015; 275:155–166.15. Dreizin D, Ahlawat S, Del Grande F, Fayad LM. Gradient-echo in-phase and opposed-phase chemical shift imaging: role in evaluating bone marrow. Clin Radiol. 2014; 69:648–657.16. Kwee TC, Takahara T, Vermoolen MA, Bierings MB, Mali WP, Nievelstein RA. Whole-body diffusion-weighted imaging for staging malignant lymphoma in children. Pediatr Radiol. 2010; 40:1592–1602. quiz 1720-172117. Padhani AR, Makris A, Gall P, Collins DJ, Tunariu N, de Bono JS. Therapy monitoring of skeletal metastases with whole-body diffusion MRI. J Magn Reson Imaging. 2014; 39:1049–1078.18. Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004; 22:275–282.19. Chen NK, Guidon A, Chang HC, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage. 2013; 72:41–47.20. Filli L, Wurnig MC, Luechinger R, Eberhardt C, Guggenberger R, Boss A. Whole-body intravoxel incoherent motion imaging. Eur Radiol. 2015; 01. 10. [Epub]. DOI: 10.1007/s00330-014-3577-z.21. Klenk C, Gawande R, Uslu L, Khurana A, Qiu D, Quon A, et al. Ionising radiation-free whole-body MRI versus (18) F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: a prospective, non-randomised, single-centre study. Lancet Oncol. 2014; 15:275–285.22. Sengupta S, Smith DS, Welch EB. Continuously moving table MRI with golden angle radial sampling. Magn Reson Med. 2014; 12. 02. [Epub]. DOI: 10.1002/mrm.25531.23. Naguib NN, Bohrt K, Nour-Eldin NE, Schulz B, Tawfik AM, Siebenhandel P, et al. Whole-body MR angiography: first experiences with the new TimCT technology with single contrast injection. J Magn Reson Imaging. 2014; 39:434–439.24. Hong TS, Greer ML, Grosse-Wortmann L, Yoo SJ, Babyn PS. Whole-body MR angiography: initial experience in imaging pediatric vasculopathy. Pediatr Radiol. 2011; 41:769–778.25. Siegel MJ, Acharyya S, Hoffer FA, Wyly JB, Friedmann AM, Snyder BS, et al. Whole-body MR imaging for staging of malignant tumors in pediatric patients: results of the American College of Radiology Imaging Network 6660 Trial. Radiology. 2013; 266:599–609.26. Hirsch FW, Sattler B, Sorge I, Kurch L, Viehweger A, Ritter L, et al. PET/MR in children. Initial clinical experience in paediatric oncology using an integrated PET/MR scanner. Pediatr Radiol. 2013; 43:860–875.27. Schäfer JF, Gatidis S, Schmidt H, Gückel B, Bezrukov I, Pfannenberg CA, et al. Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: initial results. Radiology. 2014; 273:220–231.28. Yoo HJ, Lee JS, Lee JM. Integrated whole body MR/PET: where are we? Korean J Radiol. 2015; 16:32–49.29. Atkin KL, Ditchfield MR. The role of whole-body MRI in pediatric oncology. J Pediatr Hematol Oncol. 2014; 36:342–352.30. Lee E, Goo HW, Lee JY. Age- and gender-specific estimates of cumulative CT dose over 5 years using real radiation dose tracking data in children. Pediatr Radiol. 2015; 03. 24. [Epub]. DOI: 10.1007/s00247-015-3331-y.31. Ording Müller LS, Avenarius D, Olsen OE. High signal in bone marrow at diffusion-weighted imaging with body background suppression (DWIBS) in healthy children. Pediatr Radiol. 2011; 41:221–226.32. Kellenberger CJ, Epelman M, Miller SF, Babyn PS. Fast STIR whole-body MR imaging in children. Radiographics. 2004; 24:1317–1330.33. Littooij AS, Kwee TC, Barber I, Granata C, Vermoolen MA, Enríquez G, et al. Whole-body MRI for initial staging of paediatric lymphoma: prospective comparison to an FDG-PET/CT-based reference standard. Eur Radiol. 2014; 24:1153–1165.34. Adams HJ, Kwee TC, Lokhorst HM, Westerweel PE, Fijnheer R, Kersten MJ, et al. Potential prognostic implications of whole-body bone marrow MRI in diffuse large B-cell lymphoma patients with a negative blind bone marrow biopsy. J Magn Reson Imaging. 2014; 39:1394–1400.35. Mueller WP, Melzer HI, Schmid I, Coppenrath E, Bartenstein P, Pfluger T. The diagnostic value of 18F-FDG PET and MRI in paediatric histiocytosis. Eur J Nucl Med Mol Imaging. 2013; 40:356–363.36. Miettunen PM, Lafay-Cousin L, Guilcher GM, Nettel-Aguirre A, Moorjani V. Widespread osteonecrosis in children with leukemia revealed by whole-body MRI. Clin Orthop Relat Res. 2012; 470:3587–3595.37. Cai W, Kassarjian A, Bredella MA, Harris GJ, Yoshida H, Mautner VF, et al. Tumor burden in patients with neurofibromatosis types 1 and 2 and schwannomatosis: determination on whole-body MR images. Radiology. 2009; 250:665–673.38. Monsalve J, Kapur J, Malkin D, Babyn PS. Imaging of cancer predisposition syndromes in children. Radiographics. 2011; 31:263–280.39. Yang DH, Goo HW. Generalized lymphangiomatosis: radiologic findings in three pediatric patients. Korean J Radiol. 2006; 7:287–291.40. Malattia C, Damasio MB, Madeo A, Pistorio A, Providenti A, Pederzoli S, et al. Whole-body MRI in the assessment of disease activity in juvenile dermatomyositis. Ann Rheum Dis. 2014; 73:1083–1090.41. Quijano-Roy S, Avila-Smirnow D, Carlier RY. WB-MRI muscle study group. Whole body muscle MRI protocol: pattern recognition in early onset NM disorders. Neuromuscul Disord. 2012; 22:Suppl 2. S68–S84.42. Axelsen MB, Eshed I, Duer-Jensen A, Møller JM, Pedersen SJ, Østergaard M. Whole-body MRI assessment of disease activity and structural damage in rheumatoid arthritis: first step towards an MRI joint count. Rheumatology (Oxford). 2014; 53:845–853.43. McLaughlin PD, Ryan J, Hodnett PA, O'Halloran D, Maher MM. Quantitative whole-body MRI in familial partial lipodystrophy type 2: changes in adipose tissue distribution coincide with biochemical improvement. AJR Am J Roentgenol. 2012; 199:W602–W606.44. Fritz J, Tzaribatchev N, Claussen CD, Carrino JA, Horger MS. Chronic recurrent multifocal osteomyelitis: comparison of whole-body MR imaging with radiography and correlation with clinical and laboratory data. Radiology. 2009; 252:842–851.45. Falip C, Alison M, Boutry N, Job-Deslandre C, Cotten A, Azoulay R, et al. Chronic recurrent multifocal osteomyelitis (CRMO): a longitudinal case series review. Pediatr Radiol. 2013; 43:355–375.46. Perez-Rossello JM, Connolly SA, Newton AW, Zou KH, Kleinman PK. Whole-body MRI in suspected infant abuse. AJR Am J Roentgenol. 2010; 195:744–750.47. Cha JG, Kim DH, Kim DH, Paik SH, Park JS, Park SJ, et al. Utility of postmortem autopsy via whole-body imaging: initial observations comparing MDCT and 3.0 T MRI findings with autopsy findings. Korean J Radiol. 2010; 11:395–340.48. Ross S, Ebner L, Flach P, Brodhage R, Bolliger SA, Christe A, et al. Postmortem whole-body MRI in traumatic causes of death. AJR Am J Roentgenol. 2012; 199:1186–1192.49. Ferreira EC, Brito CC, Domingues RC, Bernardes M, Marchiori E, Gasparetto EL. Whole-body MR imaging for the evaluation of McCune-albright syndrome. J Magn Reson Imaging. 2010; 31:706–710.50. Beck C, Morbach H, Wirth C, Beer M, Girschick HJ. Whole-body MRI in the childhood form of hypophosphatasia. Rheumatol Int. 2011; 31:1315–1320.51. Rittner RE, Baumann U, Laenger F, Hartung D, Rosenthal H, Hueper K. Whole-body diffusion-weighted MRI in a case of Rosai-Dorfman disease with exclusive multifocal skeletal involvement. Skeletal Radiol. 2012; 41:709–713.52. Kumar A, Goenka AH, Choudhary A, Sahu JK, Gulati S. Disseminated cysticercosis in a child: whole-body MR diagnosis with the use of parallel imaging. Pediatr Radiol. 2010; 40:223–227.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dynamic Contrast-Enhanced MRI and Its Applications in Various Central Nervous System Diseases
- Deep Learning Applications in Perfusion MRI: Recent Advances and Current Challenges
- High field strength magnetic resonance imaging of cardiovascular diseases
- Magnetic Resonance Imaging Meets Fiber Optics: a Brief Investigation of Multimodal Studies on Fiber OpticsBased Diagnostic / Therapeutic Techniques and Magnetic Resonance Imaging
- Fast MRI in Acute Ischemic Stroke: Applications of MRI Acceleration Techniques for MR-Based Comprehensive Stroke Imaging