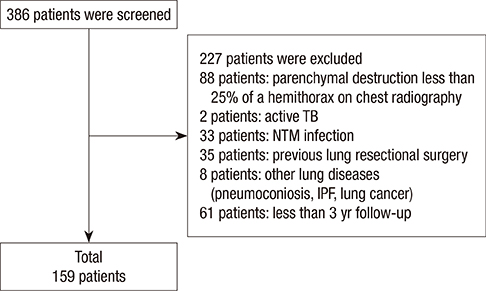
J Korean Med Sci.
2015 Jun;30(6):737-742. 10.3346/jkms.2015.30.6.737.
Effect of Airflow Limitation on Acute Exacerbations in Patients with Destroyed Lungs by Tuberculosis
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Hospital; Seoul National University College of Medicine, Seoul, Korea. cgyoo@snu.ac.kr
- KMID: 2160602
- DOI: http://doi.org/10.3346/jkms.2015.30.6.737
Abstract
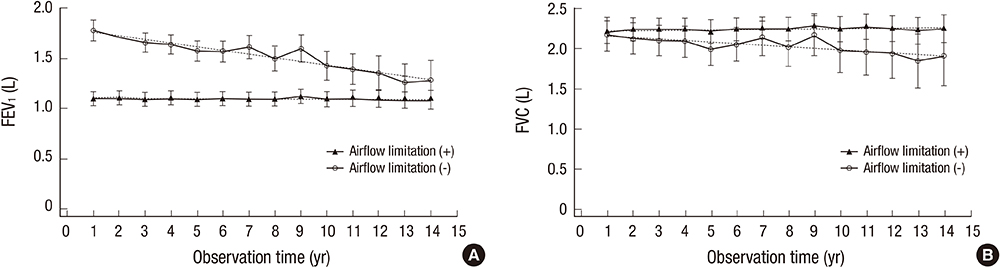
- History of treatment for tuberculosis (TB) is a risk factor for obstructive lung disease. However, it has been unclear whether the clinical characteristics of patients with destroyed lung by TB differ according to the presence or absence of airflow limitation. The objective of the study was to evaluate differences in acute exacerbations and forced expiratory volume in 1 second (FEV1) decline in patients with destroyed lung by TB according to the presence or absence of airflow limitation. We performed a retrospective cohort study and enrolled patients with destroyed lung by TB. The presence of airflow limitation was defined as FEV1/forced vital capacity (FVC) < 0.7. One hundred and fifty-nine patients were enrolled, and 128 (80.5%) had airflow limitation. The proportion of patients who experienced acute exacerbation was higher in patients with airflow limitation compared to those without (89.1 vs. 67.7%, respectively; P = 0.009). The rate of acute exacerbation was higher in patients with airflow limitation (IRR, 1.19; 95% CI, 1.11-1.27). Low body mass index (X vs. X + 1; HR, 0.944; 95% CI, 0.895-0.996) in addition to airflow limitation (HR, 1.634; 95% CI, 1.012-2.638), was an independent risk factor for acute exacerbation. The annual decline of FEV1 was 2 mL in patients with airflow limitation and 36 mL in those without (P < 0.001). In conclusion, the presence of airflow limitation is an independent risk factor for acute exacerbation in patients with the destroyed lung by TB.
MeSH Terms
Figure
Reference
-
1. Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003; 163:1009–1021.2. Pasipanodya JG, Miller TL, Vecino M, Munguia G, Garmon R, Bae S, Drewyer G, Weis SE. Pulmonary impairment after tuberculosis. Chest. 2007; 131:1817–1824.3. Plit ML, Anderson R, Van Rensburg CE, Page-Shipp L, Blott JA, Fresen JL, Feldman C. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. Eur Respir J. 1998; 12:351–356.4. Chae JN, Jung CY, Shim SW, Rho BH, Jeon YJ. CT Radiologic findings in patients with tuberculous destroyed lung and correlation with lung function. Tuberc Respir Dis. 2011; 71:202–209.5. Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000; 55:32–38.6. Lee SW, Kim YS, Kim DS, Oh YM, Lee SD. The risk of obstructive lung disease by previous pulmonary tuberculosis in a country with intermediate burden of tuberculosis. J Korean Med Sci. 2011; 26:268–273.7. Chakrabarti B, Calverley PM, Davies PD. Tuberculosis and its incidence, special nature, and relationship with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007; 2:263–272.8. Jordan TS, Spencer EM, Davies P. Tuberculosis, bronchiectasis and chronic airflow obstruction. Respirology. 2010; 15:623–628.9. Lee JH, Chang JH. Lung function in patients with chronic airflow obstruction due to tuberculous destroyed lung. Respir Med. 2003; 97:1237–1242.10. Seo YK, Lee CH, Lee HK, Lee YM, Park HK, Choi SB, Kim HG, Jang HJ, Yum HK, Lee SH. Differences between patients with TB-destroyed lung and patients with COPD admitted to the ICU. Tuberc Respir Dis. 2011; 70:323–329.11. Lam KB, Jiang CQ, Jordan RE, Miller MR, Zhang WS, Cheng KK, Lam TH, Adab P. Prior TB, smoking, and airflow obstruction: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Chest. 2010; 137:593–600.12. Perez-Padilla R, Fernandez R, Lopez Varela MV, Montes de, Muiño A, Tálamo C, Brito Jardim JR, Valdivia G, Baptista Menezes AM. Airflow obstruction in never smokers in five Latin American cities: the PLATINO study. Arch Med Res. 2012; 43:159–165.13. Caballero A, Torres-Duque CA, Jaramillo C, Bolivar F, Sanabria F, Osorio P, Orduz C, Guevara DP, Maldonado D. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study). Chest. 2008; 133:343–349.14. Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006; 61:259–266.15. Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010; 363:1128–1138.16. Cote CG, Dordelly LJ, Celli BR. Impact of COPD exacerbations on patient-centered outcomes. Chest. 2007; 131:696–704.17. Hoogendoorn M, Feenstra TL, Hoogenveen RT, Al M, Mölken MR. Association between lung function and exacerbation frequency in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2010; 5:435–444.18. Aso H, Kondoh Y, Taniguchi H, Kimura T, Nishiyama O, Kato K, Kataoka K, Hasegawa Y. Noninvasive ventilation in patients with acute exacerbation of pulmonary tuberculosis sequelae. Intern Med. 2010; 49:2077–2083.19. Celli BR, Cote CG, Marin JM, Casanova C, Montes de, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004; 350:1005–1012.20. Lainscak M, von Haehling S, Doehner W, Sarc I, Jeric T, Ziherl K, Kosnik M, Anker SD, Suskovic S. Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. J Cachexia Sarcopenia Muscle. 2011; 2:81–86.21. Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012; 7:95–99.22. Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, ECLIPSE Investigators, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011; 365:1184–1192.23. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356:775–789.24. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359:1543–1554.25. Wedzicha JA, Decramer M, Seemungal TA. The role of bronchodilator treatment in the prevention of exacerbations of COPD. Eur Respir J. 2012; 40:1545–1554.26. Jochmann A, Scherr A, Jochmann DC, Miedinger D, Török SS, Chhajed PN, Tamm M, Leuppi JD. Impact of adherence to the GOLD guidelines on symptom prevalence, lung function decline and exacerbation rate in the Swiss COPD cohort. Swiss Med Wkly. 2012; 142:w13567.27. Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009; 374:1171–1178.28. Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, Vestbo J, Knobil K, Yates JC, Calverley PM. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008; 178:332–338.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Factors Associated with Indacaterol Response in Tuberculosis-Destroyed Lung with Airflow Limitation
- Pathophysiology of Chronic Obstructive Pulmonary Disease
- Definition of Chronic Obstructive Pulmonary Disease (COPD)
- Symptom Questionnaire and Laboratory Findings in Subjects with Airflow Limitation: a Nation-wide Survey
- Physiological Basis of Tests for Airflow Limitation